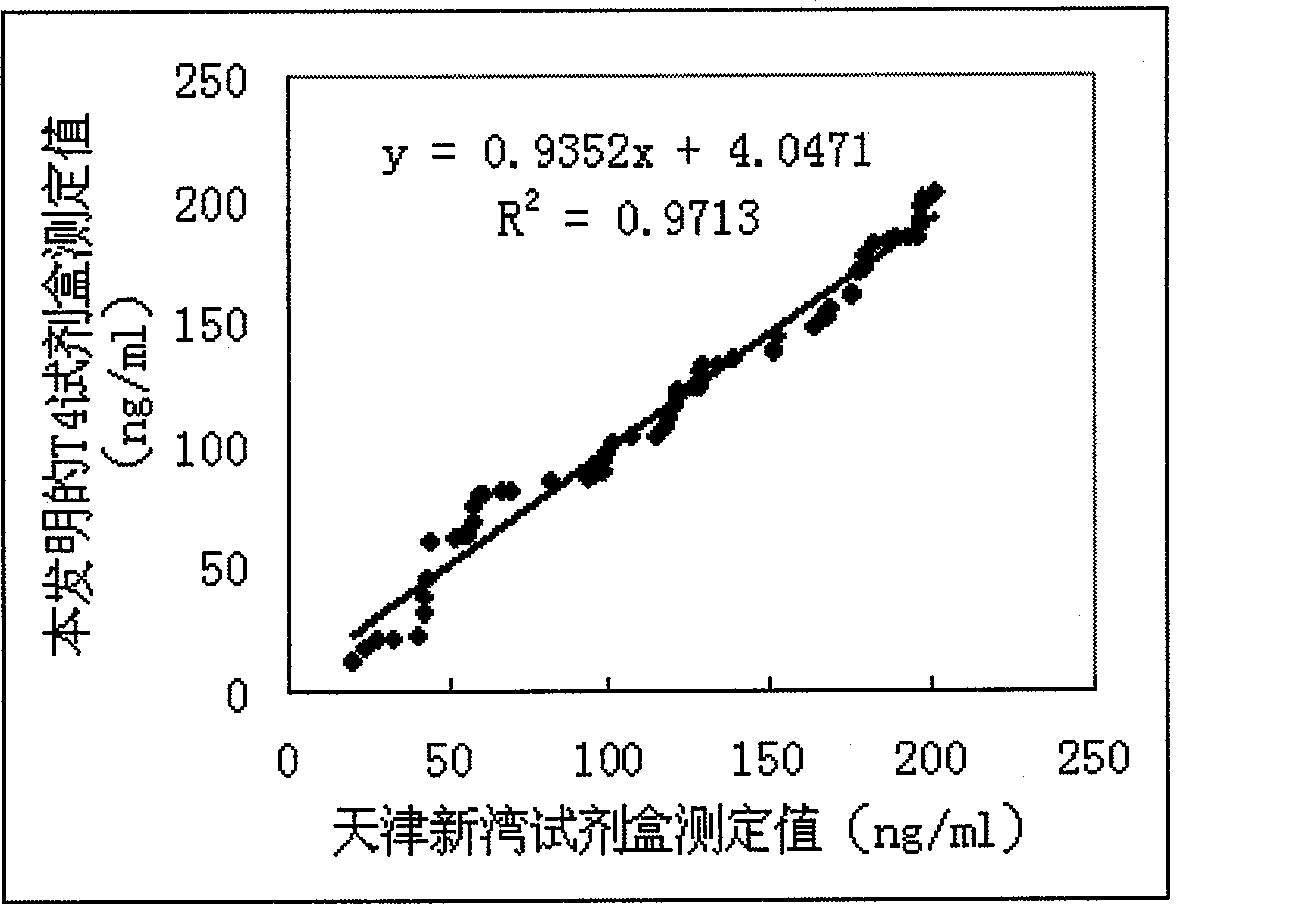
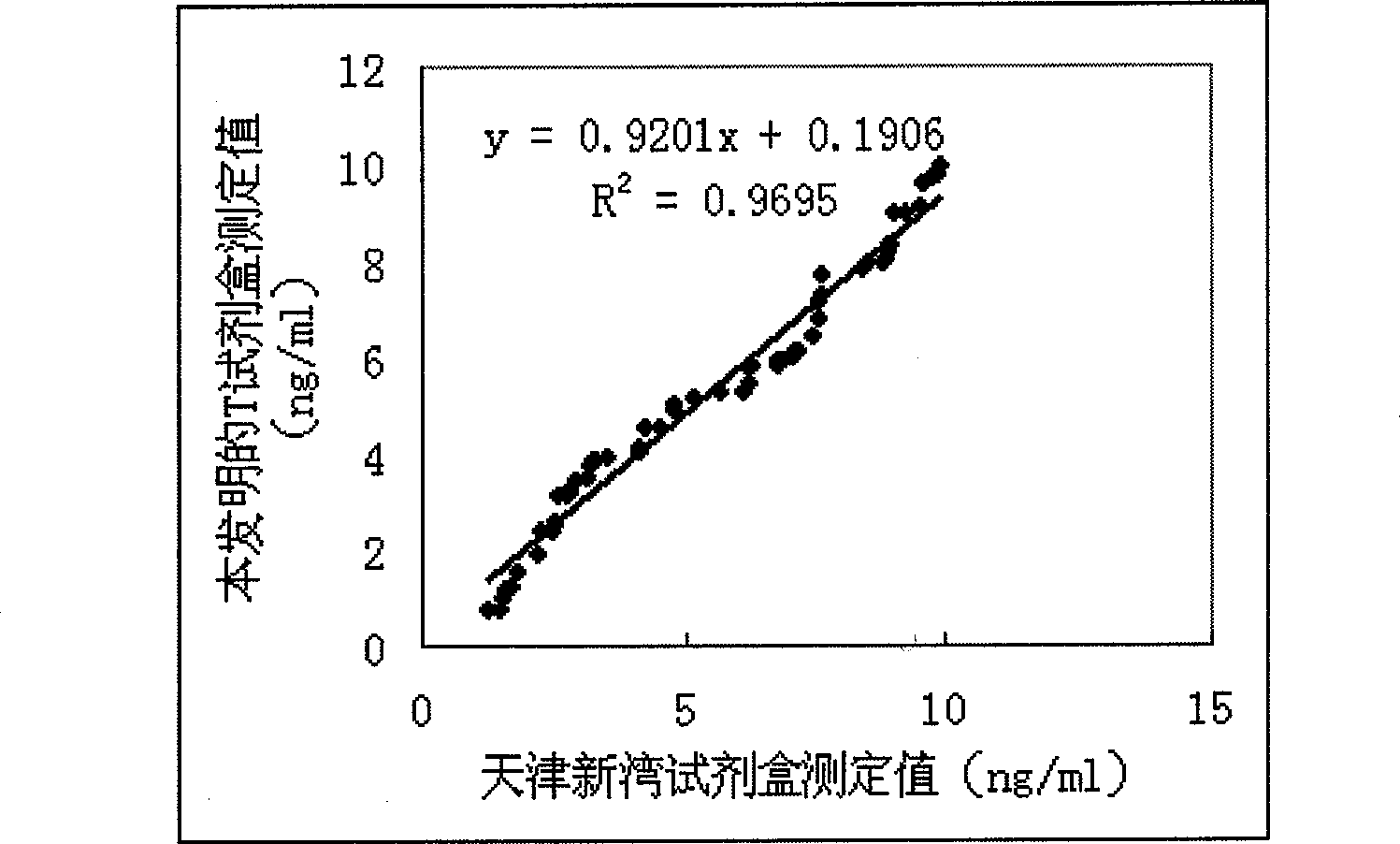
Magnetic granule competing method chemiluminescence immune analysis determination reagent kit for detecting hormone and preparing method thereof
A chemiluminescence immunology and chemiluminescence technology, which is applied in the field of immunoanalysis medicine, can solve the problems of being unable to be widely used in clinical diagnosis and scientific research work, and restricting popularization and use, so as to achieve the effects of ensuring sensitivity, improving sensitivity, and facilitating operation and production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Preparation of Thyroxine (T4) Magnetic Particle Competition Chemiluminescent Immunoassay Quantitative Assay Kit of the Present Invention (Using Alkaline Phosphatase to Prepare Labels)
[0041] 1. Marker preparation
[0042] Alkaline phosphatase was used to label the T4 antigen using the glutaraldehyde method, and the specific labeling process was as follows:
[0043] Mix according to the ratio of alkaline phosphatase: T4 antigen = 1:2, add glutaraldehyde for labeling with a volume of 10% of the mixed solution, mix well and react at room temperature for 1-2 hours. The solution was transferred into a dialysis bag, dialyzed overnight with 50 mM PBS solution of pH 7.4, and the medium was changed twice in the middle. Transfer the solution into a glass bottle, add an equal amount of high-quality glycerin, mix well, and store it below -20°C.
[0044] 2. Preparation of T4 calibrator
[0045] Prepared with T4 pure product, divided into 6 bottles of 0, 10, 25, 50, 15...
Embodiment 2
[0080] Example 2 Preparation of Thyroxine (T4) Magnetic Particle Competition Chemiluminescence Immunoassay Quantitative Assay Kit of the present invention (Horseradish Peroxidase is used to prepare markers)
[0081] The horseradish peroxidase-labeled T4 antigen adopts the sodium periodate oxidation method, and the specific labeling process is as follows:
[0082] Weigh 5 mg of HRP and dissolve in 1 mL of double distilled water. Add 0.2 mL of freshly prepared 0.1M NaIO to the above solution 4 The solution was stirred at room temperature in the dark for 20 minutes. Add 20 μL of 0.2M carbonate buffer at pH 9.5 to raise the pH of the above-formylated HRP to 9.0-9.5, then immediately add 10 mg of T4 antigen in 1 mL of 0.01M carbonate buffer, and gently Stir for 2 hours. Put the above solution into a dialysis bag, and dialyze against 0.15 M pH7.4 PBS at 4° C. overnight. Centrifuge at 10,000rpm for 30 minutes to remove the precipitate. The supernatant is the enzyme conjugate. Add...
Embodiment 3
[0084] Example 3 Preparation of the testosterone (T) magnetic particle competition method chemiluminescent immunoassay quantitative assay kit of the present invention
[0085] Except that the T antibody was used to coat the magnetic particles, the enzyme labeled T antigen was used to prepare the enzyme marker, and the concentration of the calibrator was 0, 0.5, 1.0, 2.5, 10, 20 ng / mL, the same method as in Example 1 and 2 was used to prepare T Chemiluminescent Immunoassay Quantitative Assay Kit for Magnetic Particle Competition Method.
[0086] The prepared test kit is tested by conventional manufacturing and testing procedures in this area, and the results are shown in the following table 2:
[0087] Table 2
[0088] Test items Inspection standards test result accuracy The average recovery rate is 90.0-110.0% 106.5% specificity Cross-reaction rate with its analogues ≤0.01% 0.003% Precision CV(%) ≤15%(n=10) 6% sensitivity ≤0.20ng / mL...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com