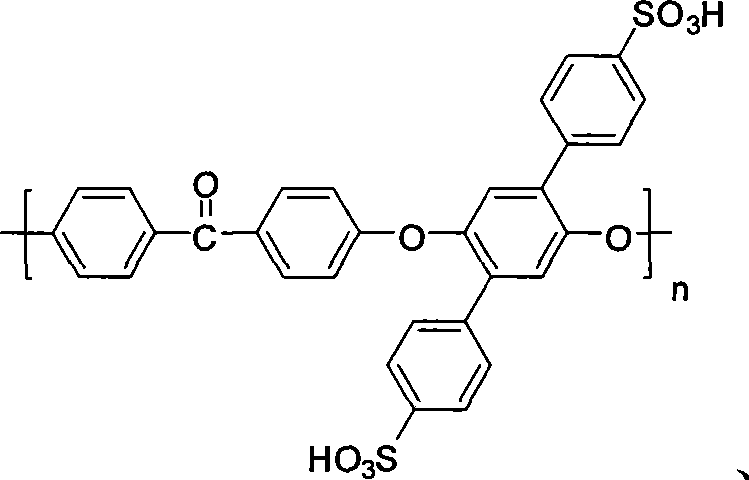
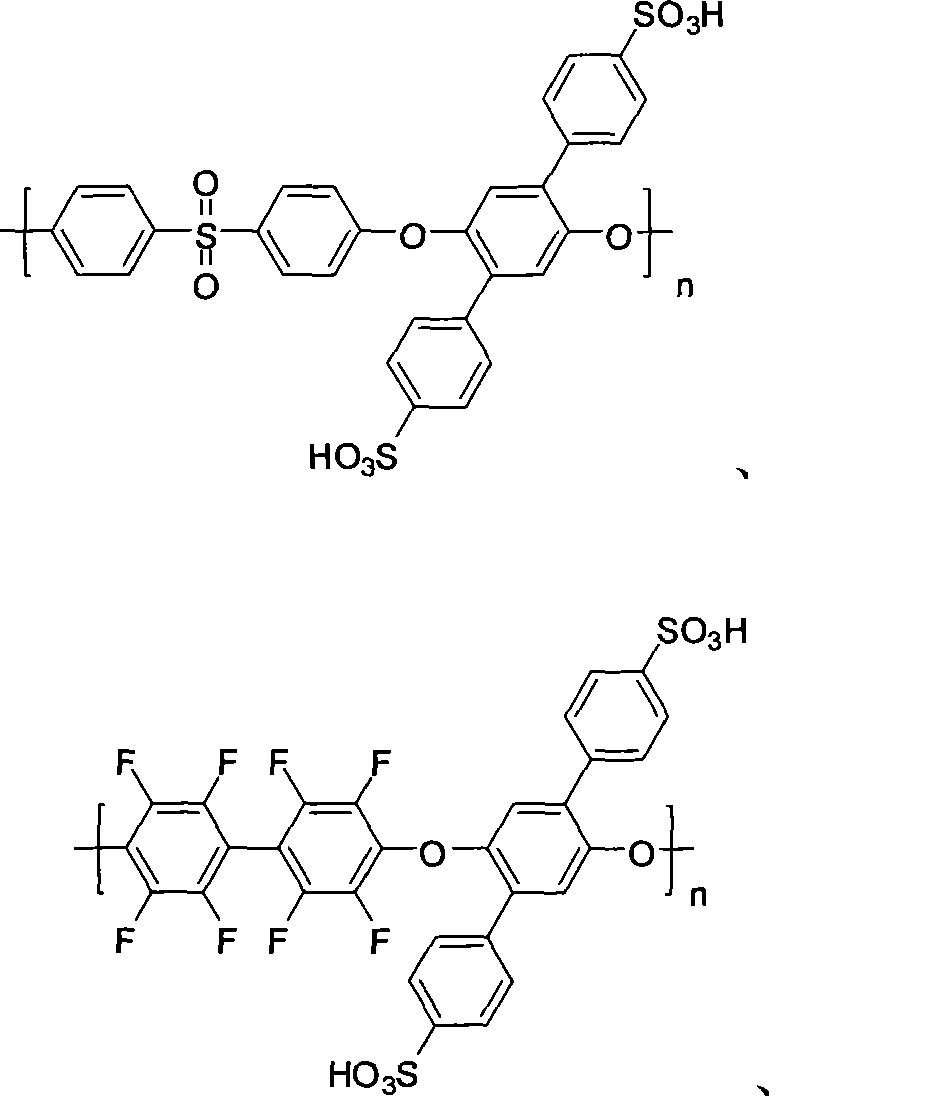
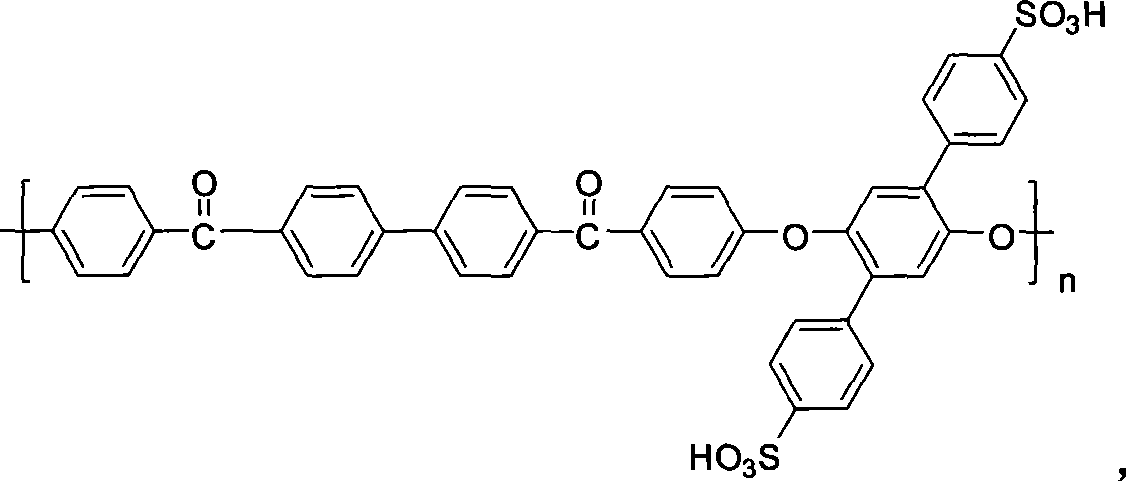
Polyarylether material bearing a sulfonic acid group on a pendant group, and preparation method and application thereof
A technology of side group sulfonic acid and polyarylether, which is applied in the field of high thermal stability side group sulfonic acid type polyarylether polymer and its preparation, can solve the problems such as the degree of sulfonation and the difficult position of sulfonation, and avoid cumbersome And complex synthesis, good thermal stability, wide application temperature effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the preparation of 2,5-diphenylhydroquinone
[0037] Put 26 grams (0.1mol) of 2,5-diphenylbenzoquinone, 65.4 grams (1.0mol) of zinc powder, and 200 mL of water into a three-necked flask equipped with mechanical stirring, dropping funnel and reflux condenser, and 160 mL of concentrated hydrochloric acid Drop in at a rate of 0.04mL / sec. Reaction at reflux temperature for 8 hours, hot filtration to obtain product 2,5-diphenylhydroquinone, recrystallized, yield 60%, melting point 226°C.
[0038] The process is the same as above, and the zinc powder is fed in batches, which can be divided into 5 batches of 13 grams each to obtain the same product with a yield of 65% and a melting point of 226°C.
Embodiment 2
[0039] Example 2: Initial Polymer - Synthesis of Homopolymer DiPh-PES-100
[0040] 2.62 grams (0.01mol) of 2,5-diphenylhydroquinone, 2.54 grams (0.01mol) of 4,4'-difluorodiphenyl sulfone and 1.8 grams of anhydrous potassium carbonate were prepared by the method of Example 1 , put 20mL N, N-dimethylacetamide, 30ml toluene into a 100ml three-necked flask equipped with a water dispenser, pass nitrogen gas, heat up to toluene reflux and stir, reflux for 1.5 to 2 hours, remove toluene, heat up to 220°C, After continuing to react for 4-8 hours, the polymerization solution was precipitated in water, crushed, washed and dried to obtain the initial polymer DiPh-PES-100.
Embodiment 3
[0041] Example 3: Synthesis of initial polymer - copolymer DiPh-PES-50
[0042]2.62 grams (0.01mol) of 2,5-diphenylhydroquinone, 2.50 grams (0.01mol) of 4,4'-dihydroxydiphenyl sulfone, 5.08 grams (0.02mol) 4, Put 4'-difluorodiphenyl sulfone and 1.8 grams of anhydrous potassium carbonate, 40 mL of N, N-dimethylacetamide, and 30 mL of toluene into a 500-mL three-neck flask equipped with a water dispenser, ventilate nitrogen, and heat up to toluene Reflux and stir for 1.5 to 2 hours, remove toluene, raise the temperature to 190°C, and continue the reaction for 4 to 8 hours. The polymerization solution is precipitated in water, crushed, washed and dried to obtain the initial polymer DiPh-PES-50. Its glass transition temperature (Tg) is 225°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com