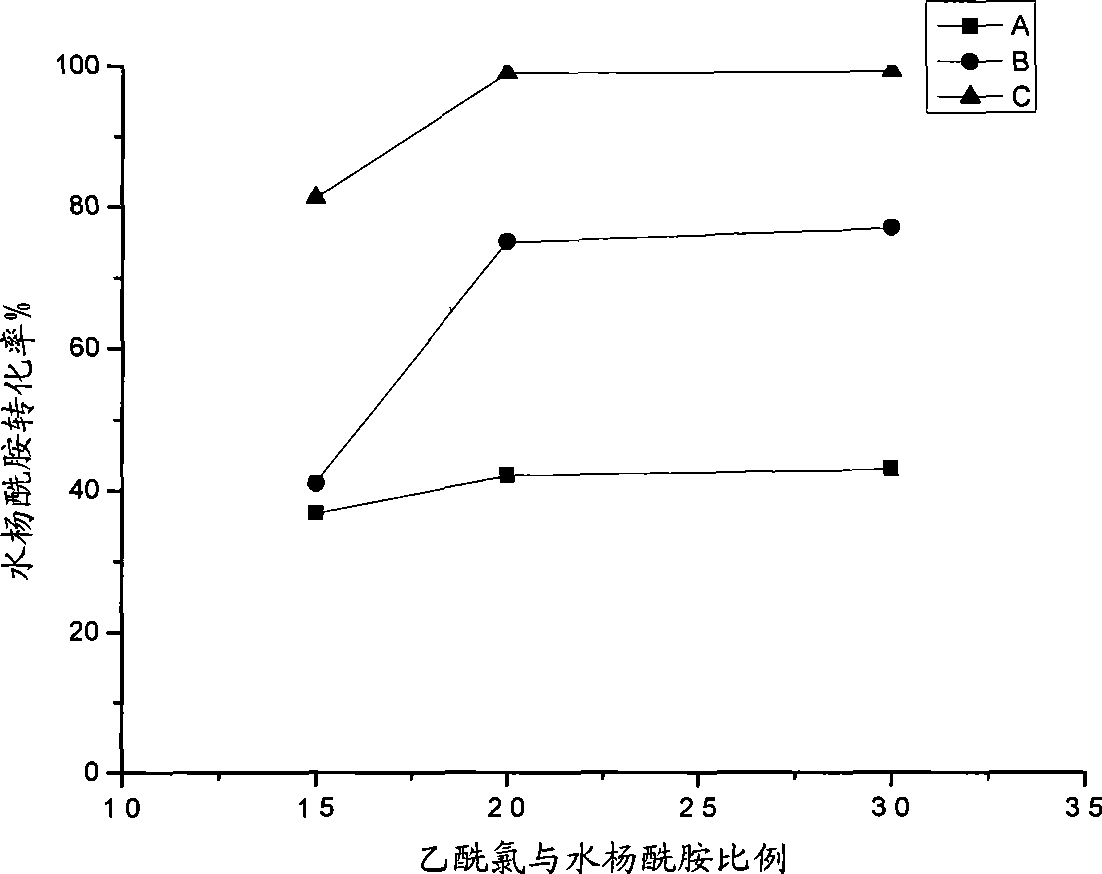
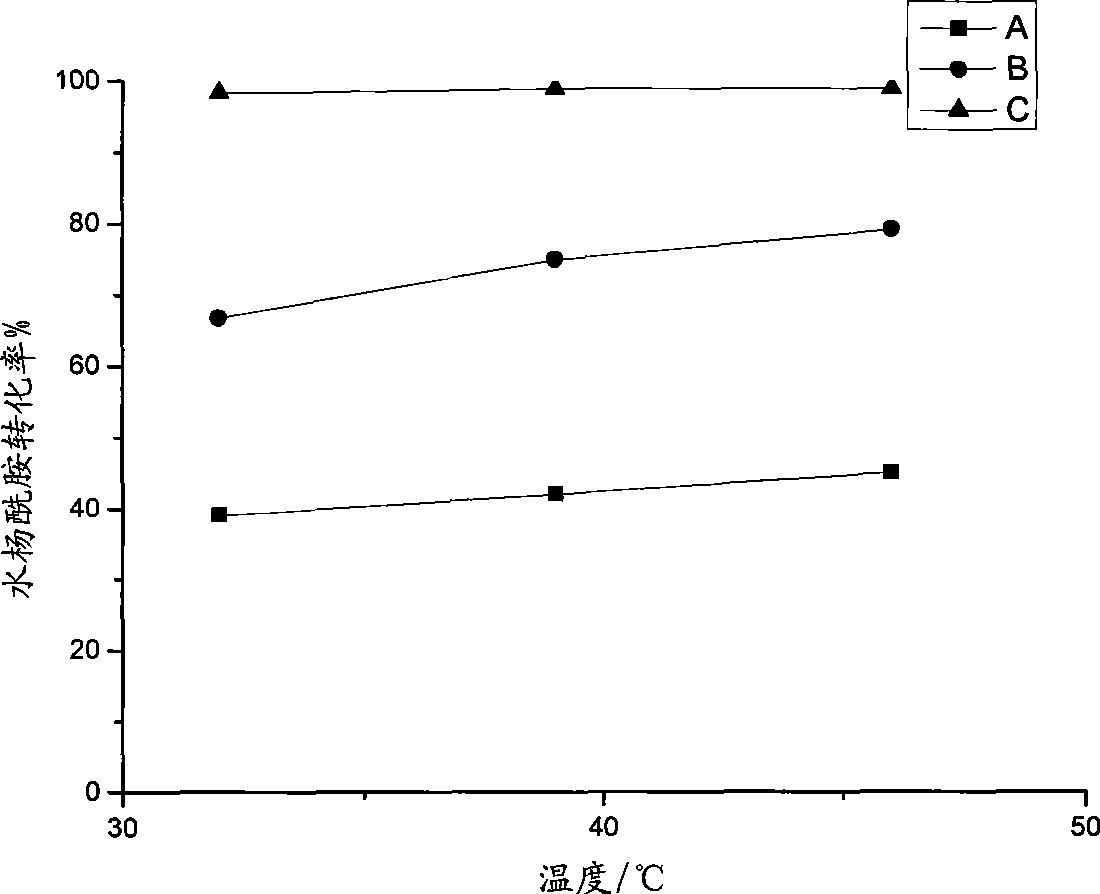
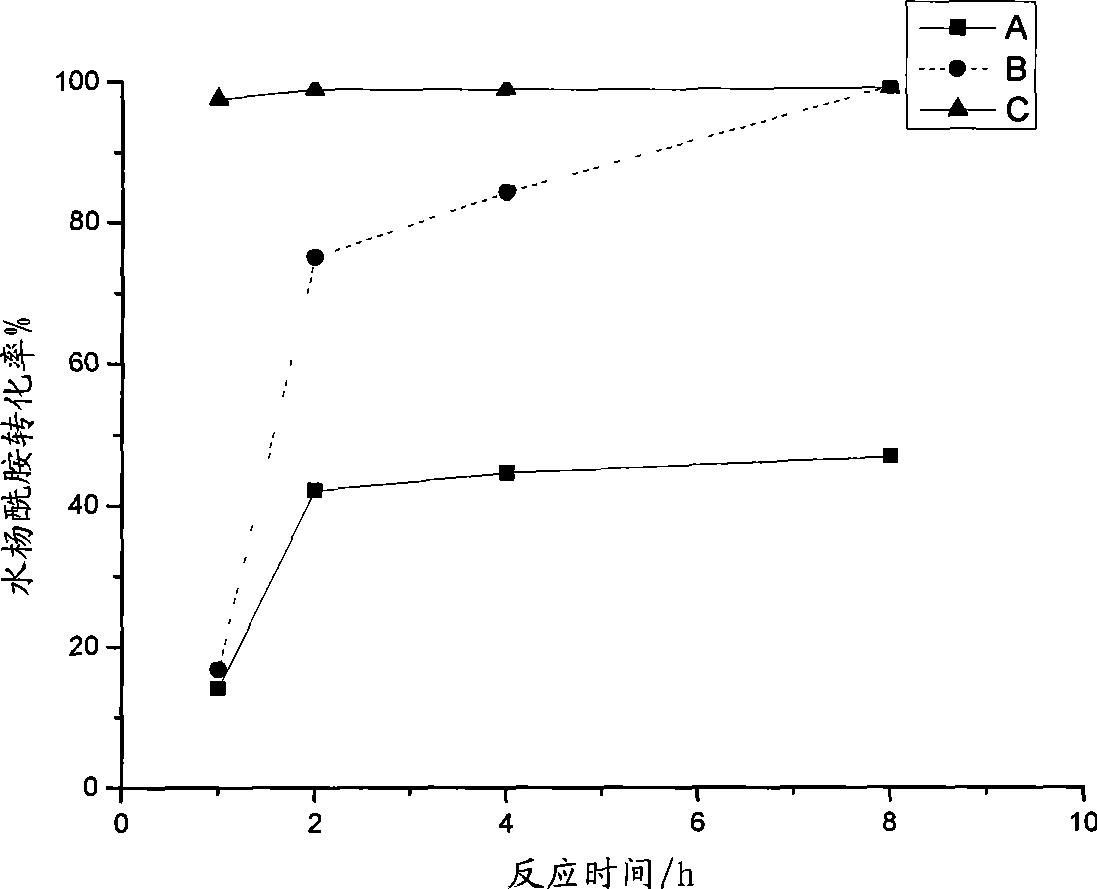
Method for preparing acetsalicylamide
A technology of acetylsalicylamide and salicylamide, which is applied in the field of preparation of acetylsalicylamide, can solve problems such as no research report on acetylsalicylamide, and achieve low risk and toxicity, good selectivity, and high conversion rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of ionic liquids
[0024] Pour triethylamine into a clean 250mL large-necked flask in a fume hood, cover with a stopper, and place the flask in an ice-water bath for cooling. Quickly add a small amount of concentrated hydrochloric acid to the flask several times, stir well to make the reaction complete and the temperature will not be too high. Due to the high toxicity of triethylamine, in order to ensure that triethylamine is fully neutralized, a slight excess of concentrated hydrochloric acid needs to be added. Stir until the solution is a single homogeneous phase and keep for 10 min. After the neutralization reaction was completed, the solution was rotated to remove most of the water under vacuum, and triethylamine hydrochloride was crystallized to obtain a white solid, which was dried at 110° C. for 24 hours for use.
[0025] Charge dry nitrogen in the anaerobic and anaerobic operation box, take the triethylamine hydrochloride prepared above in a 250mL ...
Embodiment 2
[0032] The preparation of ionic liquid is with embodiment 1.
[0033] Charge nitrogen into a 100mL dry three-neck round bottom flask, add 6ml (about 20mmol) of ionic liquid catalyst, and turn on reflux condensed water; ℃, 46℃; after the salicylamide and the ionic liquid are mixed into a uniform slurry, add 200% theoretical amount of acetyl chloride under stirring, stir well to make the mixture uniform, and start to calculate the reaction time for 2 hours. After the predetermined reaction time is reached, the heating is stopped. Quickly add 50mL of dilute hydrochloric acid (diluted 10-20 times with concentrated hydrochloric acid) to the above-mentioned three-necked round-bottomed flask to fully hydrolyze the catalyst and unreacted acetyl chloride, stir and cool to about 30°C, and precipitate solids. Transfer to a 100mL small beaker and age for 2 days. Suction filtration, washing with hydrochloric acid solution, 20 mL each time, repeated 3 times, and then washing with distille...
Embodiment 3
[0036]The preparation of ionic liquid is with embodiment 1.
[0037] In a dry 100mL three-necked round-bottomed flask, fill nitrogen, add 6ml (about 20mmol) of ionic liquid catalyst, and turn on reflux condensed water; then add 20mmol, 15mmol, and 10mmol salicylamide under stirring, and slowly heat up to 39°C; After salicylamide and ionic liquid are mixed into a uniform slurry, add 200% theoretical amount of acetyl chloride under stirring, stir well to mix evenly, and start to calculate the reaction time 1h (60min), 2h, 4h, 8h (480min) . After the predetermined reaction time is reached, the heating is stopped. Quickly add 50mL of dilute hydrochloric acid (diluted 10-20 times with concentrated hydrochloric acid) to the above-mentioned three-necked round-bottomed flask to fully hydrolyze the catalyst and unreacted acetyl chloride, stir and cool to about 30°C, and precipitate solids. Transfer to a 100mL small beaker and age for 2 days. Suction filtration, washing with hydrochl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com