Equine influenza detection kit and detection method
A technology of equine influenza and kits, applied in biochemical equipment and methods, microbiological determination/inspection, DNA/RNA fragments, etc., can solve the problem of inability to calculate the starting DNA or RNA copy number, inability to achieve accurate quantification, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] Embodiment 1: the preparation and use of kit
[0136] 1. See Table 3 for the composition of the kit.
[0137] Table 3 Kit preparation composition
[0138] Composition (48tests / box) quantity lysate 5.0mL×6 tubes H3 and N8 Subtype Equine Influenza Virus Fluorescence
RT-PCR Single Reaction Solution Each 750μL×1 tube Fluorescence RT-PCR of H3 N8 Subtype Equine Influenza Virus
double reaction solution
750μL×1 tube Taq enzyme (5U / μL) 45μL×1 tube RT-PCR enzyme particles 1 capsule x 36 tubes DEPC water 1mL×3 tubes negative control 1mL×3 tubes positive control 1mL×3 tubes
[0139] 2. How to use the kit
[0140] 2.1 RNA extraction:
[0141] Take n 1.5ml sterilized centrifuge tubes (n = number of samples + 1 tube of negative control + 1 tube of positive control) and mark them. First add 600ul of lysate (the lysate is highly corrosive, do not touch the skin or clothes, otherwise rinse with plenty...
Embodiment 2
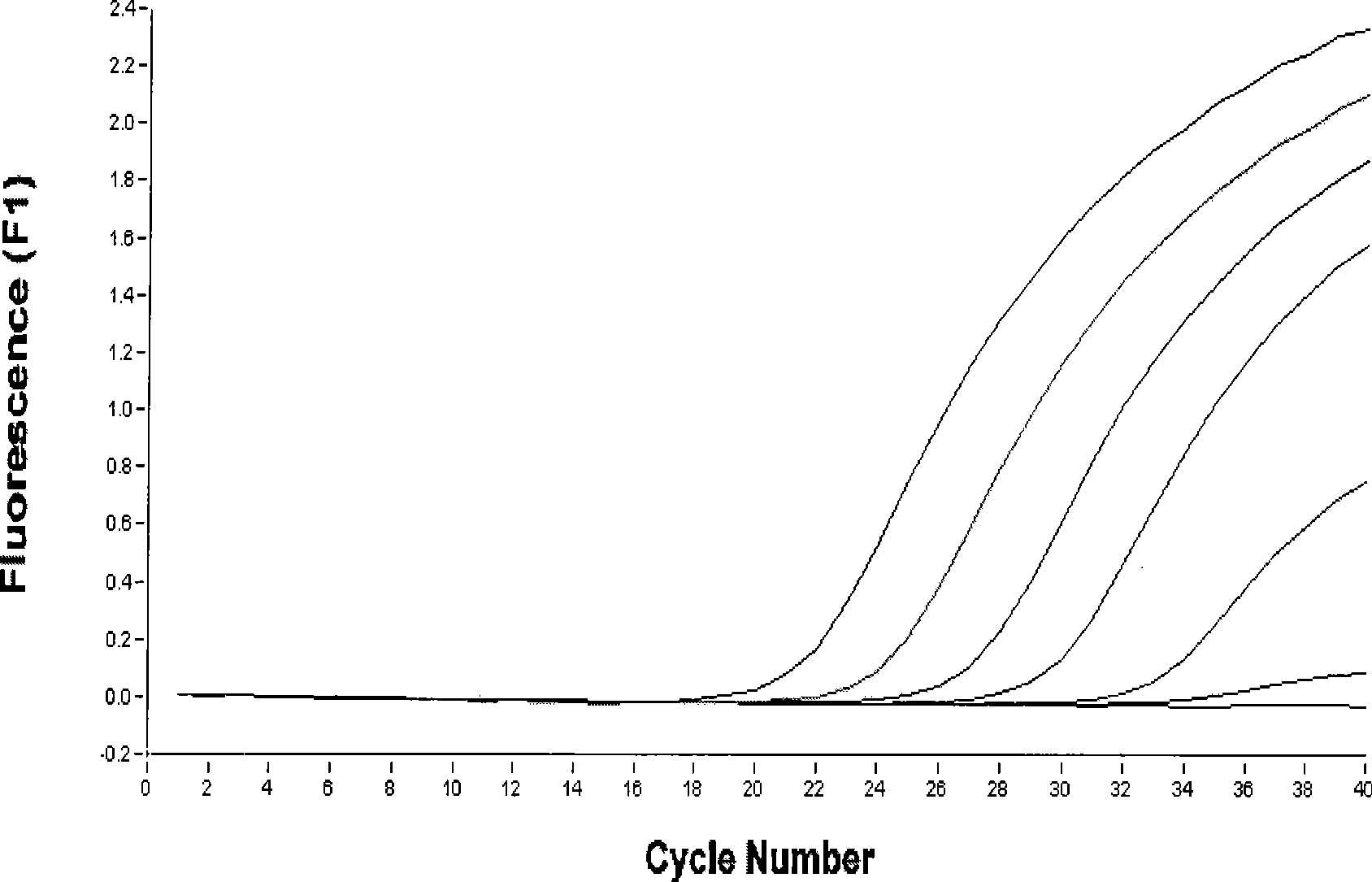
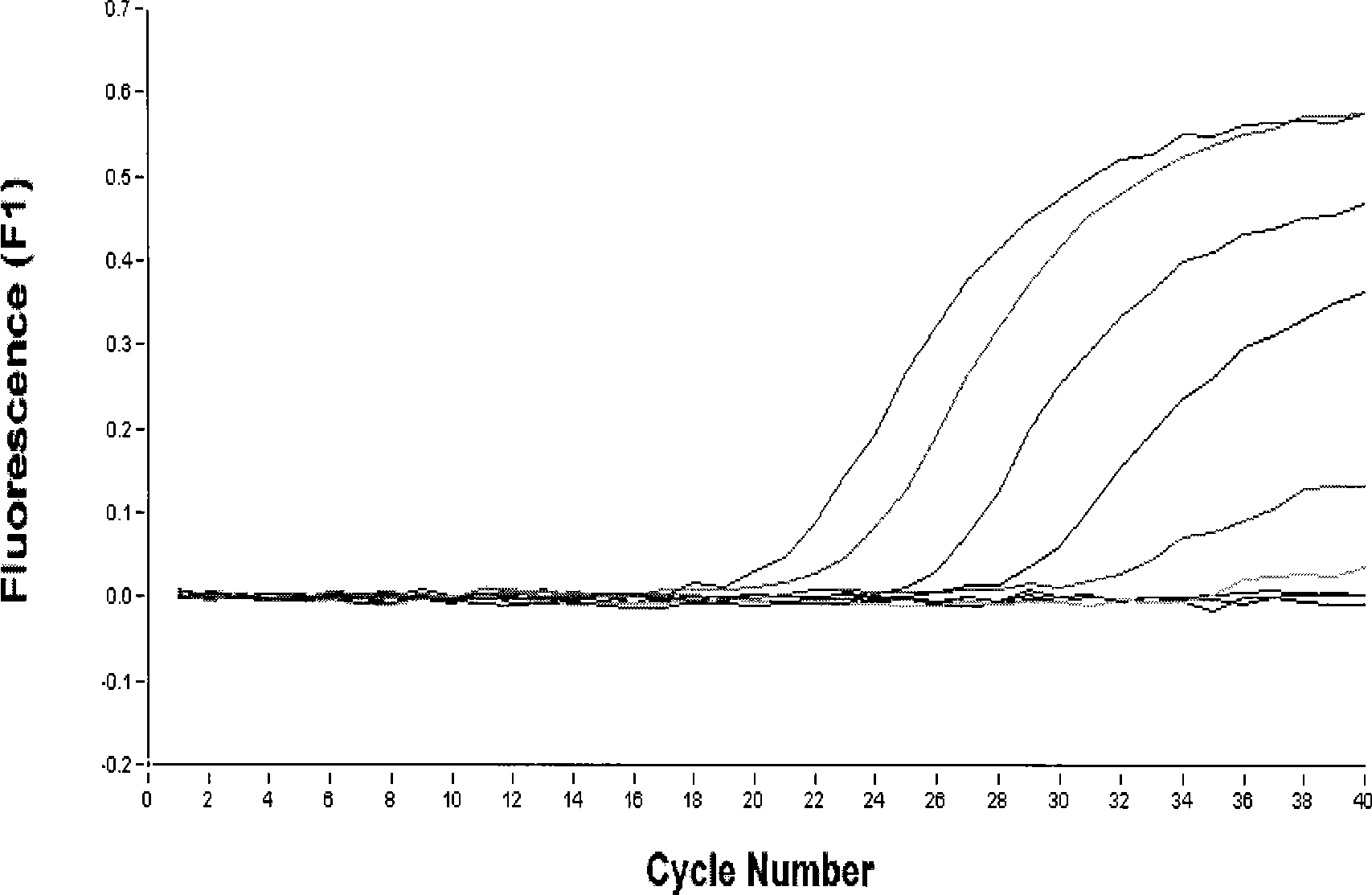
[0153] Embodiment 2: the sensitivity test of kit and be used for the relative quantification of virus RNA
[0154] 1. Materials:
[0155] Methods The virus strain used in the research process was the H3N8 subtype equine influenza virus A / equine / xibei / 1 / 2007 (10 -5 EID50 / 0.1ml).
[0156] 2. Method
[0157] 1) The allantoic fluid of H3N8 subtype equine influenza virus A / equine / xibei / 1 / 2007 chick embryo culture was used as 10 -1 、10 -2 、10 -3 、10 -4 、10 -5 、10 -6 、10 -7 、10 -8 、10 -9 、10 -10 Fluorescence RT-PCR detection of H3 and N8 single and double H3N8 subtype equine influenza viruses were performed respectively, and the viruses of each dilution were inoculated into SPF chicken embryos, and the sensitivity of the two methods was compared.
[0158] 2) Relative quantification of viral RNA:
[0159] In order to achieve the relative quantification of the amount of viral RNA in clinical samples, we further detected the serial 10-fold dilution of viral RNA samples, per...
Embodiment 3
[0163] Embodiment 3: the specificity test of kit
[0164] 1. Materials
[0165] Table 4 Virus strains applied in the method research process
[0166]
[0167] 2. Method
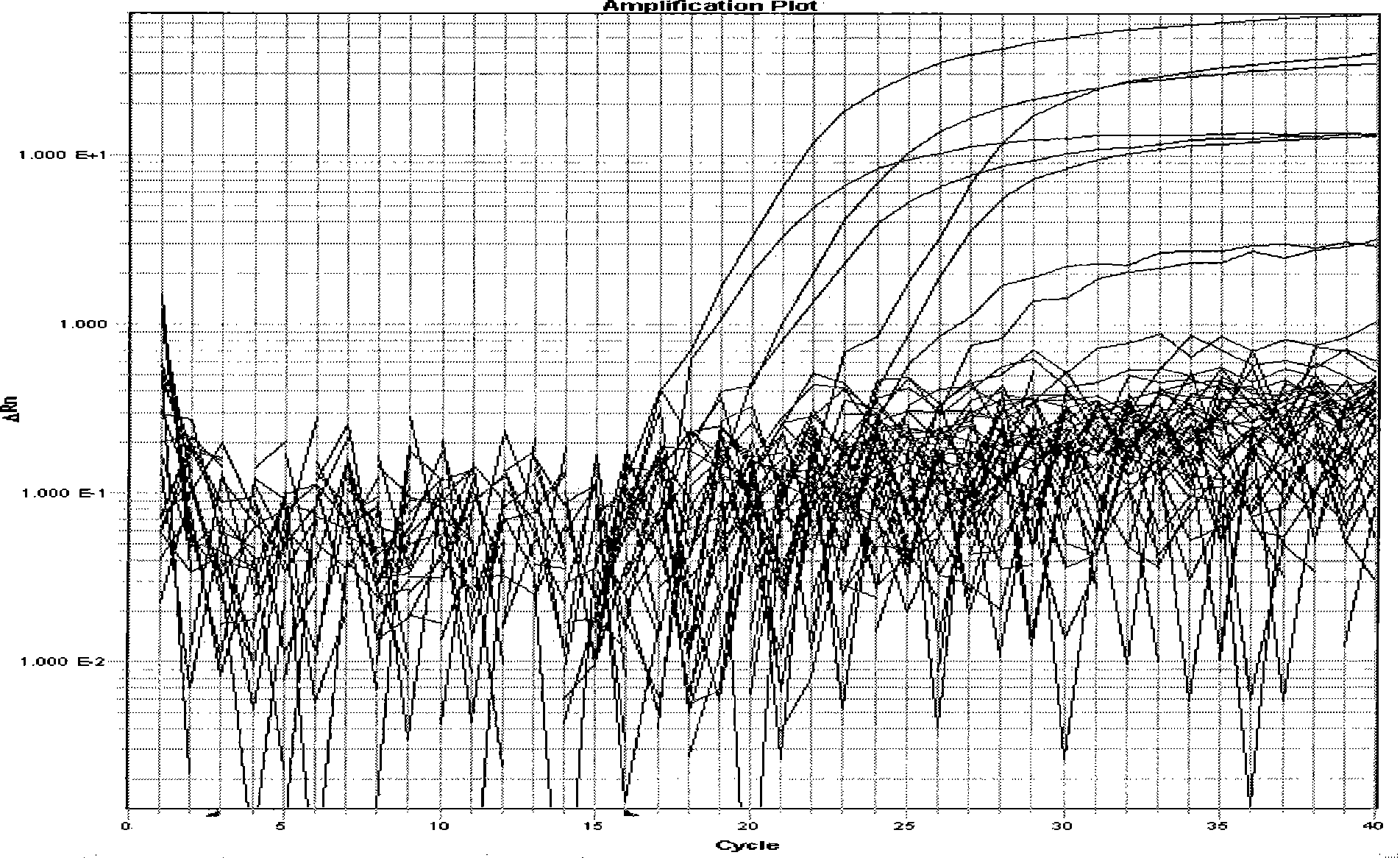
[0168] Use the established single and double fluorescent RT-PCR method to detect a variety of influenza viruses (including H1, avian H3, H5, H9 subtype viruses), equine arteritis virus, and vest type 1 influenza virus to verify the method specificity.
[0169] 3. Results
[0170] Figure 5 and Figure 6 As shown, the results showed that the established method had no cross-reaction with the above viruses and had good specificity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com