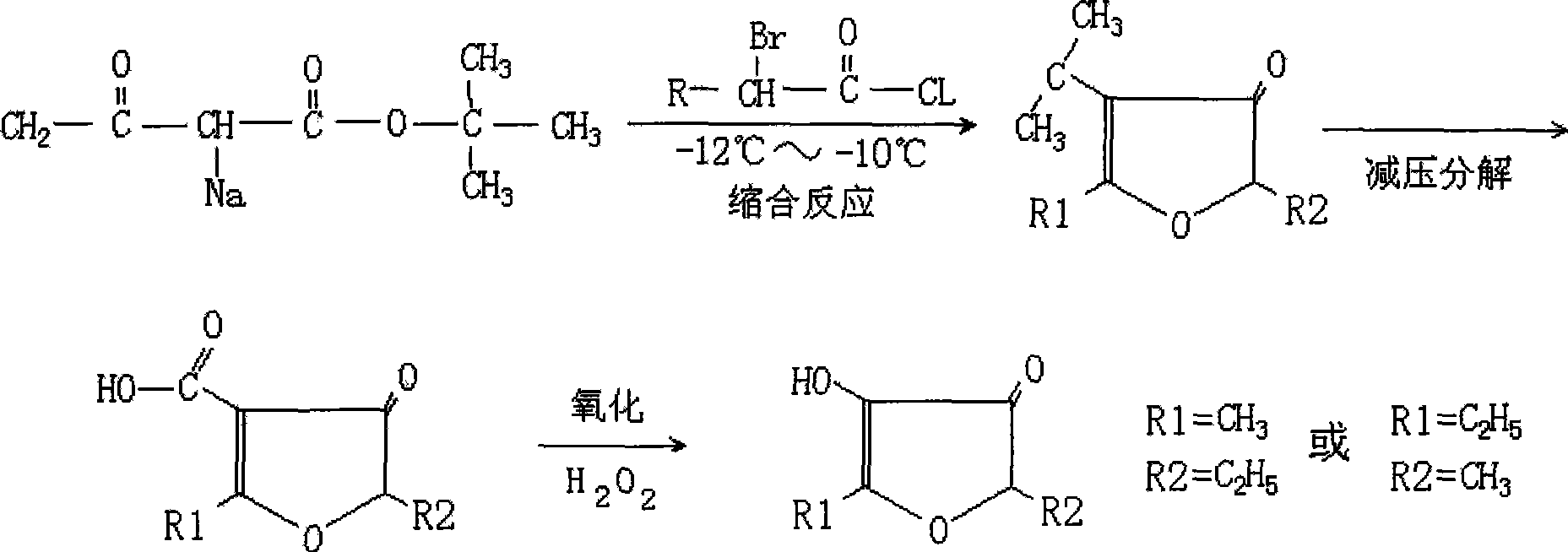
Method for preparing alkyl furanone by low-temperature condensation method
A technology of alkyl furanone and condensation method, applied in organic chemistry and other directions, can solve the problems of long process cycle, high toxicity of raw materials, complicated process, etc., and achieve the effects of short process cycle, ideal yield and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Preparation of tert-butyl sodium acetoacetate
[0030] Add 3000ml of anhydrous diethyl ether in the three-necked flask, then put 150 grams (6.52mol) of sodium metal, then dropwise add 1135 grams (7.18mol) of tert-butyl acetoacetate, the rate of addition is until the diethyl ether refluxes, and maintains reflux for 3 Hours, the metal sodium was fully reacted, and the reaction product was white and thick sodium tert-butyl acetoacetate.
[0031] 2. Preparation of furan ring
[0032] In a three-necked flask equipped with a thermometer, mechanical stirring, and a dropping funnel, add white thick tert-butyl sodium acetoacetate, and add 600 grams (3.24mol) of α-bromobutyryl chloride dropwise to tert-butyl sodium acetoacetate. In the ester, the temperature of the reactant is controlled at -10°C during the dropwise addition process, and the temperature is maintained for 1 hour after the addition, and then placed at room temperature for 12 hours, and 900ml of water is added t...
Embodiment 2
[0038] 1. Preparation of tert-butyl sodium acetoacetate
[0039] Add 1500ml of anhydrous ether in the three-necked flask, then put 100 grams (4.35mol) of sodium metal, and then dropwise add 757 grams (4.79mol) of tert-butyl acetoacetate, the rate of addition is until the ether is refluxed, and the reflux is maintained for 5 minutes after adding. Hours, the metal sodium was fully reacted, and the reaction product was white and thick sodium tert-butyl acetoacetate.
[0040] 2. Preparation of furan ring
[0041] In a three-necked flask equipped with mechanical stirring, a thermometer, and a dropping funnel, add white thick tert-butyl sodium acetoacetate, and add 402 grams (2.17mol) of α-bromobutyryl chloride dropwise to tert-butyl sodium acetoacetate. In the butyl ester, the temperature of the reactant is controlled at -12°C during the dropwise addition process, and the reaction is maintained at this temperature for 2 hours after the addition, and then placed at room temperature...
Embodiment 3
[0047] 1. Preparation of tert-butyl sodium acetoacetate
[0048] Add 3000ml of anhydrous ether in the there-necked flask, then put 125 grams (5.43mol) of sodium metal, then dropwise add 945 grams (5.98mol) of tert-butyl acetoacetate, the rate of addition is so far that the ether is refluxed, and the reflux is maintained for 4 hours after adding. Hours, the metal sodium was fully reacted, and the reaction product was white and thick sodium tert-butyl acetoacetate.
[0049] 2. Preparation of furan ring
[0050] In a three-necked flask equipped with a thermometer, mechanical stirring, and a dropping funnel, add white thick tert-butyl sodium acetoacetate, and add 500 grams (2.7mol) of α-bromobutyryl chloride dropwise to tert-butyl sodium acetoacetate. In the ester, the temperature of the reactant is controlled at -11°C during the dropwise addition process, and the temperature is maintained for 1 hour after the addition, and then placed at room temperature for 10 hours, and 1000ml o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com