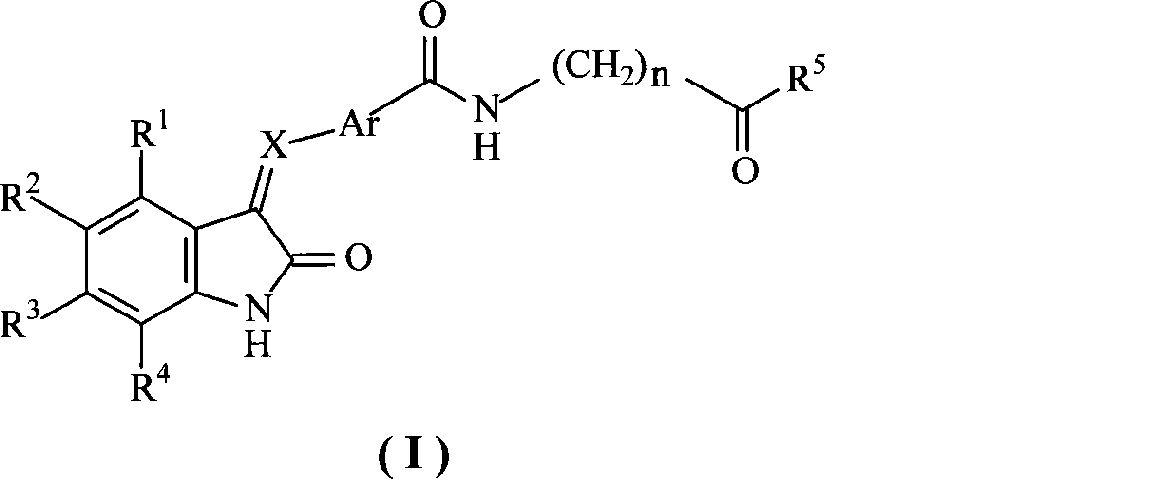
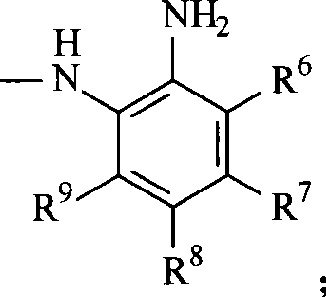
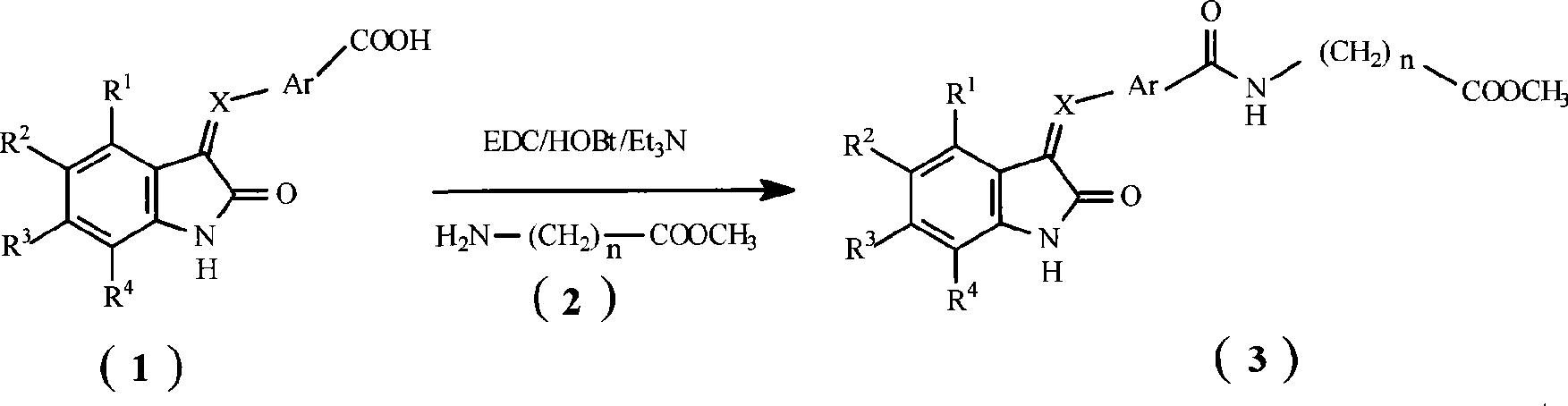
2-dihydroindolemanone derivates as histone deacetylase inhibitor, preparation method and use thereof
A technology of indolinone and derivatives is applied to 2-indolinone derivatives as histone deacetylase inhibitors, its preparation method and application field, and can solve the problems of ineffective curative effect on solid tumors and the like, achieve excellent curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] 4-((5-fluoro-2-oxo-1,2-dihydro-indol-3-ylidene)methyl)benzoic acid and
[0068] Preparation of 3-((5-fluoro-2-oxo-1,2-dihydro-indol-3-ylidene)methyl)benzoic acid
[0069]
[0070]Add 1.51g (10mmol) 5-fluoro-2-indolinone (US7,179,910 B2, Examplel), 1.50g (10mmol) 4-carboxybenzaldehyde (purchased from Alfa Aesar company), 1.28g (15mmol) in the reaction flask ) piperidine and 25ml of absolute ethanol, heated to reflux for 6 hours, cooled to room temperature, and filtered. Transfer the filter cake into a beaker, add 100ml of water, neutralize to pH=4 with 2M hydrochloric acid, collect the solid by filtration, wash the solid with water, and dry in vacuo to obtain a brown solid 4-((5-fluoro-2-oxo-1,2 - Dihydro-indol-3-ylidene)methyl)benzoic acid (1.33 g, 47% yield). LC-MS (m / z) 284 (M+1).
[0071] Using 5-fluoro-2-indolinone and 3-carboxybenzaldehyde (purchased from Alfa Aesar) as raw materials, 3-((5-fluoro-2-oxo-1,2-dihydro- Indol-3-ylidene)methyl)benzoic acid, LC-MS...
Embodiment 2
[0073] 5-(((5-fluoro-2-oxo-1,2-dihydro-indol-3-ylidene)hydrazono)methyl)-
[0074] Preparation of 2,4-dimethyl-1H-pyrrole-3-carboxylic acid and other compounds
[0075]
[0076]
[0077] Add 8.25g (50mmol) 5-fluoroisatin (purchased from Aldrich) and 150ml 50% hydrazine hydrate into the reaction bottle, react at 80-90°C for 3 hours, cool to room temperature, filter, wash with water, collect the solid, and dry it in vacuum to obtain Yellow solid 5-fluoro-2-oxo-1,2-dihydro-indole-3-ylidenehydrazine (6.0 g, 67% yield). LC-MS (m / z) 180 (M+1).
[0078] Add 1.79g (10mmol) 5-fluoro-2-oxo-1,2-dihydro-indole-3-ylidene hydrazine, 1.67g (10mmol) 5-formyl-2,4-dimethyl Base-1H-pyrrole-3-carboxylic acid (US 7,179,910 B2, Examplel), 1.28g (15mmol) piperidine and 25ml absolute ethanol, heated to reflux for 6 hours, cooled to room temperature, and filtered. Transfer the filter cake to a beaker, add 100ml of water, neutralize to pH=4 with 2M hydrochloric acid, collect the solid by filtr...
Embodiment 3
[0087] 2-(5-(((5-fluoro-2-oxo-1,2-dihydro-indol-3-ylidene)hydrazono)methyl)-2,4-dimethyl-
[0088] Preparation of 1H-pyrrole-3-amido)methyl acetate
[0089]
[0090] 328 mg (1 mmol) of 5-(((5-fluoro-2-oxo-1,2-dihydro-indol-3-ylidene)hydrazono)methyl)-2,4-dimethyl-1H -Pyrrole-3-carboxylic acid was dissolved in 8 ml of DMF, then 384 mg (2 mmol) of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride, 162 mg (1.2 mmol) of 1 - Hydroxybenzotriazole, 404 mg (4 mmol) triethylamine and 151.8 mg (1.2 mmol) glycine methyl ester hydrochloride. The mixture was stirred at room temperature for 24 hours. Then dilute with 400ml saline. The solid was collected by vacuum filtration. The solid was washed with water. Dry in vacuo to give red solid 2-(5-(((5-fluoro-2-oxo-1,2-dihydro-indol-3-ylidene)hydrazono)methyl)-2,4-dimethyl (244 mg, 61% yield). LC-MS (m / z) 400 (M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com