Method for preparing direct methanol fuel cell carbon-carried Pt-based catalyst
A methanol fuel cell and catalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, battery electrodes, etc., can solve problems such as complicated operation, reduced catalyst performance, and increased risk, and achieve simple, safe, and good operation Effect of Electrocatalytic Methanol Oxidation Activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] First, disperse 100 mg of carbon nanotubes treated with nitric acid in 50 ml of twice distilled water, disperse ultrasonically, and add 6.6 mL of 19.3 mmol / LH 2 PtCl 6 Aqueous solution, continue to ultrasonically disperse for 30min, stir magnetically at 70°C to volatilize the solvent water to obtain a dry powder, disperse the obtained dry powder with 30ml ethylene glycol, add an excess of 0.2mol / L NaBH at 60°C 4 The aqueous solution is reduced, centrifuged, washed and dried to obtain a Pt / CNTs catalyst with a loading capacity of 20%.
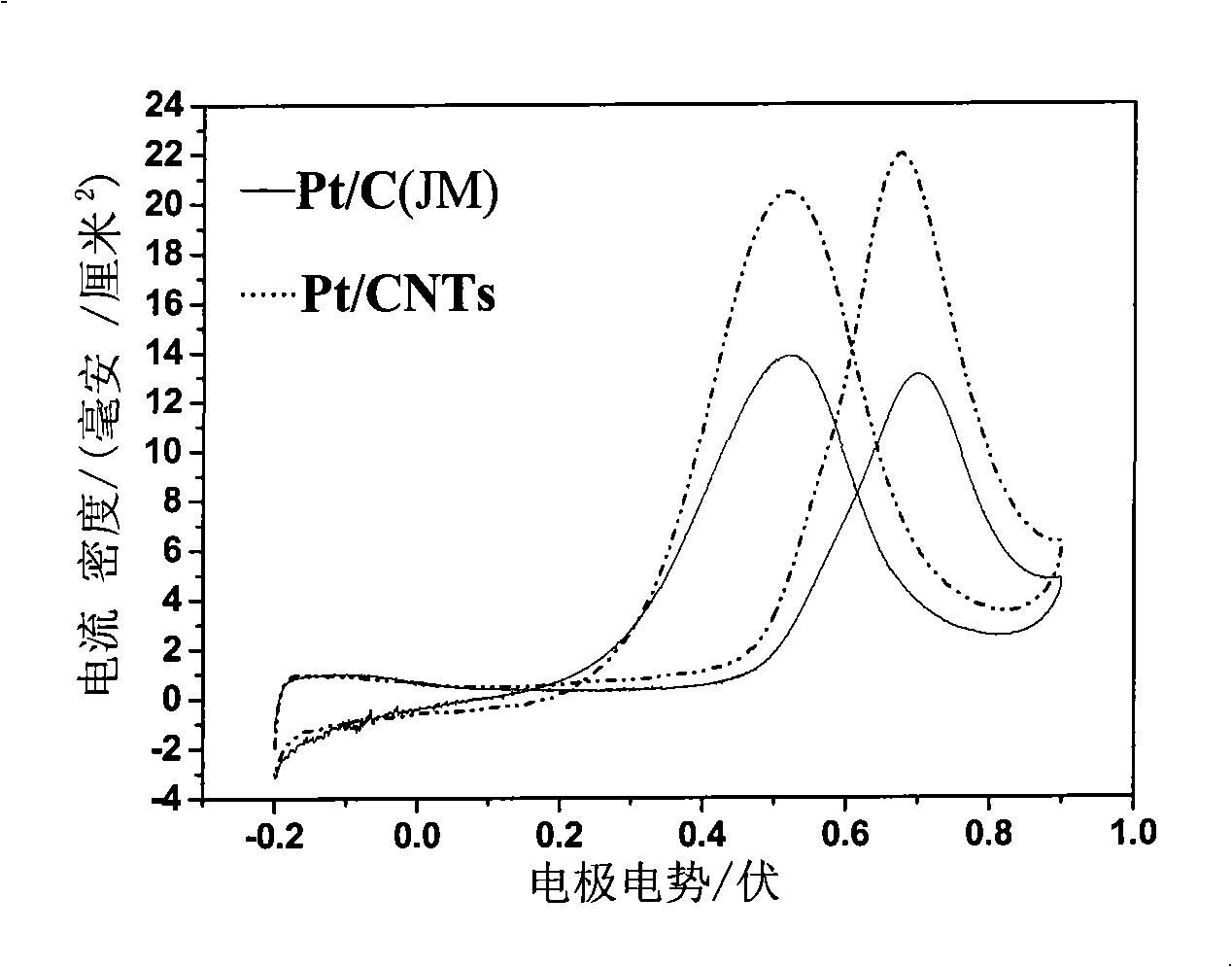
[0023] figure 1 It is the comparative diagram of the catalytic oxidation of methanol by the 20% Pt / CNTs catalyst prepared in Example 1 and the 20% Pt / C (JM) catalyst produced and sold by Johnson Matthey Company, and the reference electrode is Ag-AgCl. Depend on figure 1 It can be seen that the carbon-supported Pt-based metal catalyst prepared in this example exhibits higher methanol catalytic performance, and the positive sweep current...
Embodiment 2
[0025] This embodiment is the same as embodiment 1 except the following features: add 3.3mL 19.3mmol / L H dropwise 2 PtCl 6 Aqueous solution, the loading capacity is 10% Pt / CNTs catalyst.
Embodiment 3
[0027] This embodiment is the same as embodiment 1 except the following features: add 6.6mL19.3mmol / LH dropwise 2 PtCl 6 and 4.3ml 28.9mmol / LRuCl 3 Aqueous solution, ultrasonic dispersion. A catalyst with a loading of 20%Pt10%Ru / CNTs (Pt:Ru≈1:1) was obtained.
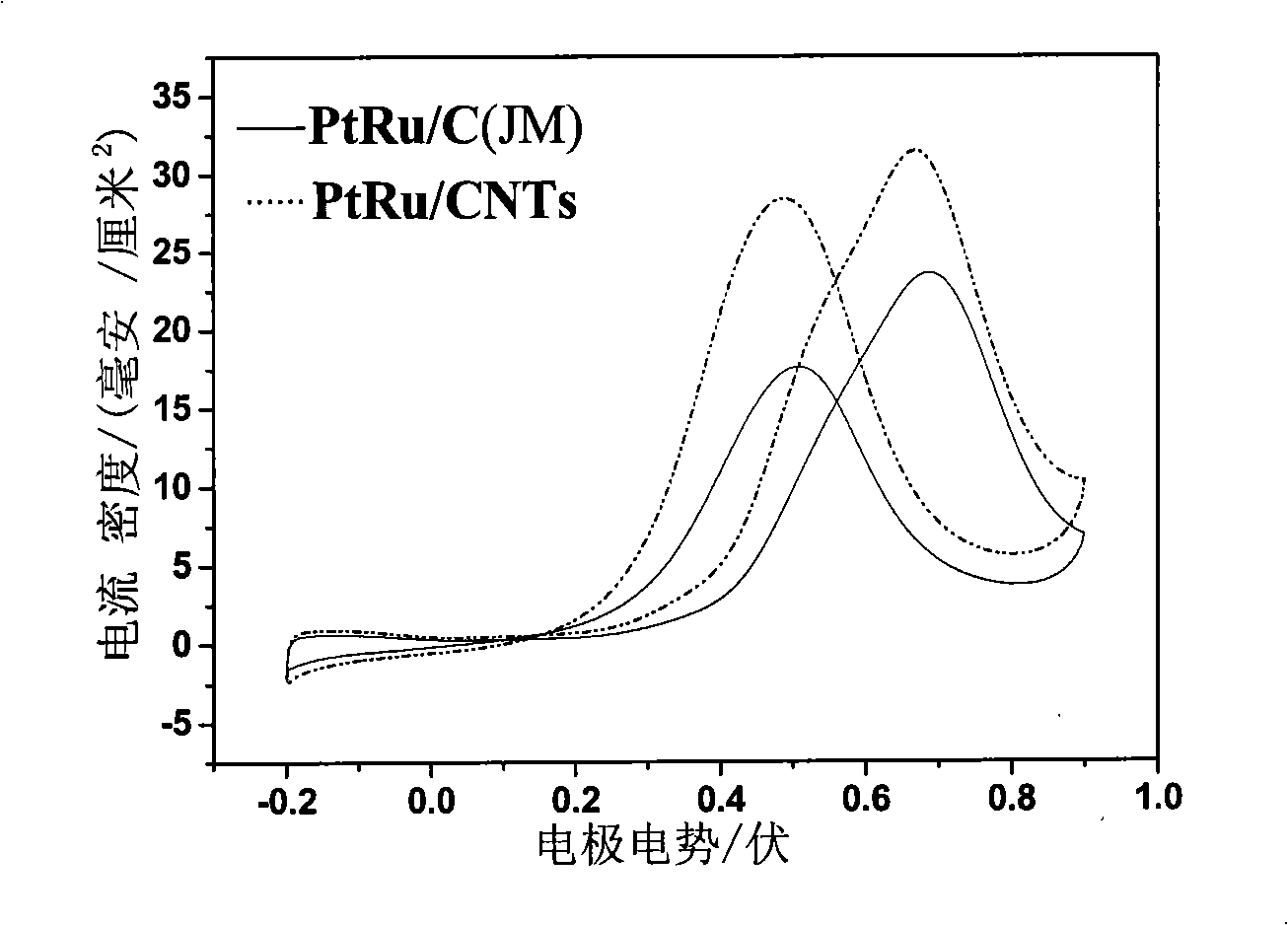
[0028] figure 2 The 20%Pt10%Ru / C (JM) catalyst that is the Pt-Ru / CNTs that embodiment 3 prepares and Johnson Matthey company produces and sells in 1mol / LCH 3 OH+0.5mol / LH 2 SO 4 In the cyclic voltammetry curve, the reference electrode is Ag-AgCl. Depend on figure 2 It can be seen that the Pt-Ru / CNTs catalyst has a higher catalytic methanol oxidation peak current than the commercial catalyst.
[0029] Figure 4 For the XRD curves of the Pt / CNTs prepared in Example 1 and the Pt-Ru / CNTs prepared in Example 3, the diffraction peak of carbon (002) appeared at the 2θ angle of 24.6°. Among them, the (111), (200), (220), (311) crystal plane diffraction peaks of Pt appear in the Pt / CNTs curve at 39.8°, 46.1°, 67.5° a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com