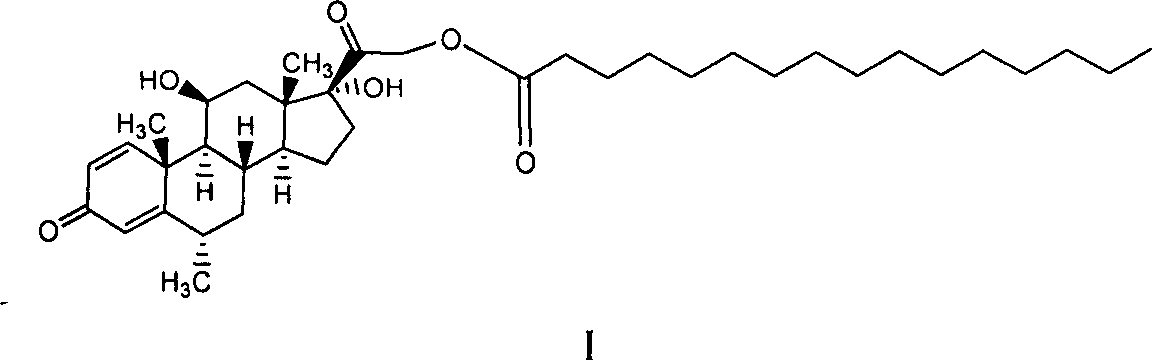
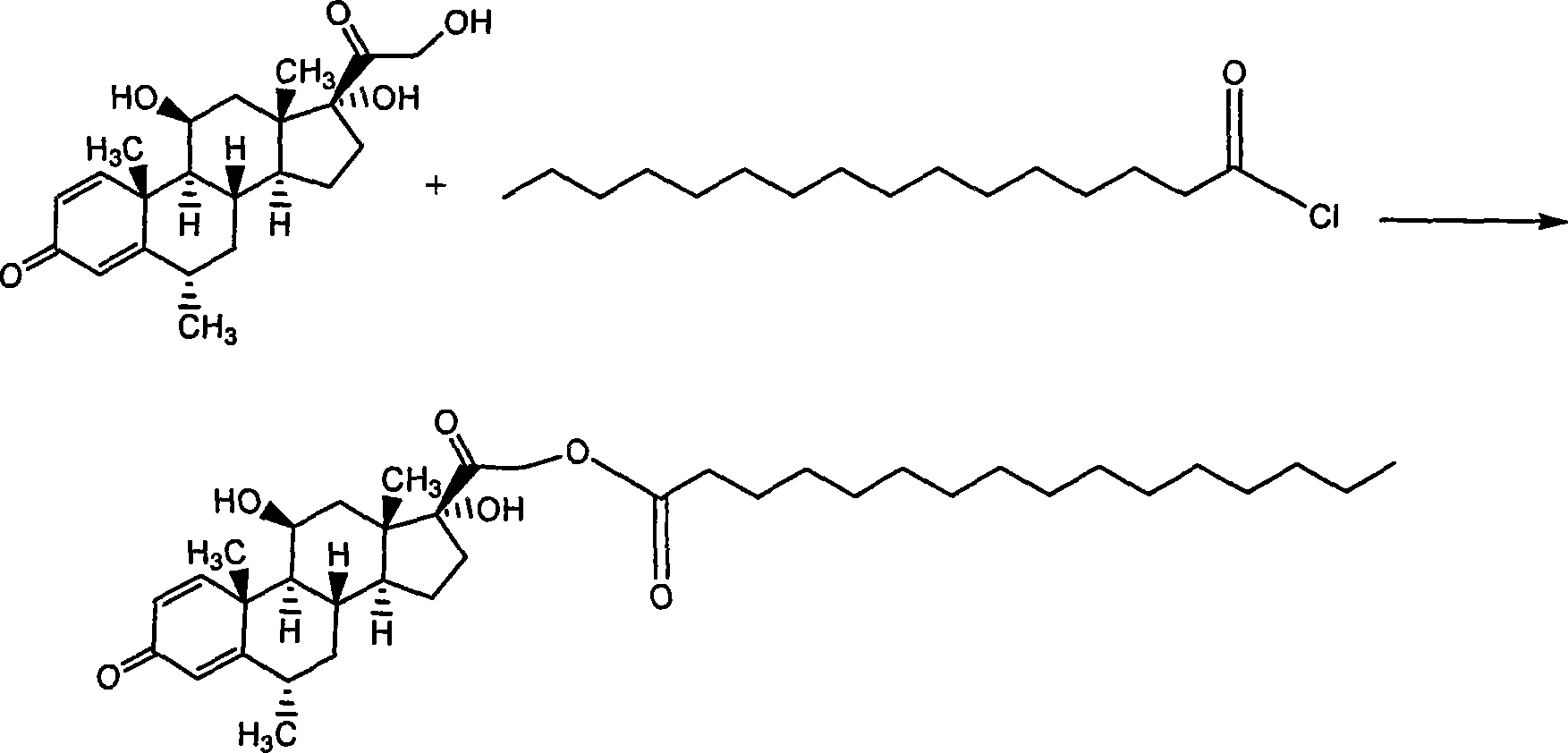
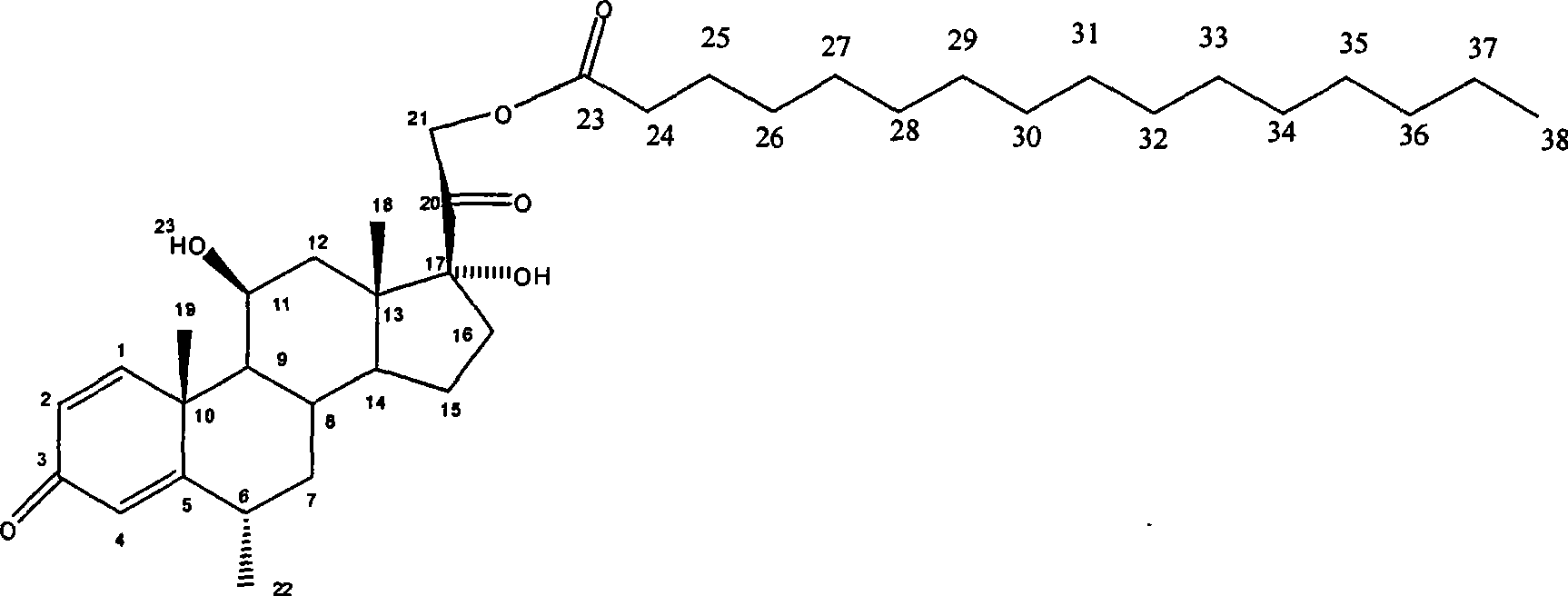
Composition with methylprednisolone palmitate as active component for treating local inflammation
A kind of technology of methylprednisolone and palmitate, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1 (lyophilized emulsion)
[0040] Methylprednisolone palmitate 2.5g (Methylprednisolone is about 1.5g),
[0041] Soy Lecithin 10g
[0042] Anhydrous sodium bisulfite 0.01g
[0043] The remaining amount of water for injection to 100ml
[0044] Process:
[0045] 1) In the preparation equipment, dissolve 2.5 g of methylprednisolone palmitate in an appropriate amount of ethanol;
[0046] 2) Mix 10g of soybean lecithin for injection with an appropriate amount of water for injection, and add 0.01g of vitamin C
[0047] 3) Add the mixture obtained in 2) into the mixture obtained in 1) under the condition of stirring, after stirring at 20°C, pass through a homogenizer to homogenize the solution repeatedly to obtain a uniform solution, and prepare 100ml of liquid medicine , add 6g of glucose as a freeze-drying protective agent, pass through a 0.22μm microporous membrane to sterilize, and pack aseptically under 100 grades.
[0048] 4) remove moisture through free...
Embodiment 2
[0050] Embodiment 2 (lyophilized emulsion)
[0051] Methylprednisolone palmitate 1.7g (about 1g methylprednisolone)
[0053] Tea oil for injection 2g
[0054] Anhydrous sodium bisulfite 0.01g
[0055] Ethylparaben 0.01g
[0056] Glucose 5g
[0057] Water for injection to 100ml
[0058] craft
[0059] 1) In the preparation equipment, 1.7 g of methylprednisolone palmitate was dissolved in 2 g of tea oil for injection;
[0060] 2) Mix 2 g of egg yolk lecithin for injection with an appropriate amount of water for injection, and add 0.01 g of ethylparaben and 0.01 g of anhydrous sodium bisulfite.
[0061] 3) Add the mixture obtained in step 1) to the mixture obtained in step (2) under stirring conditions, stir and pass through a homogenizer at 40°C, homogenize the solution repeatedly to obtain a uniform solution, and adjust the pH to 6 with potassium acetate ~7, Add glucose as a freeze-drying protective agent to prepare 100ml of liquid medicine...
Embodiment 3
[0064] Embodiment 3 (lyophilized emulsion)
[0065] Methylprednisolone palmitate 4g (about 2.5g methylprednisolone)
[0066] Poloxamer 4g
[0067] Olive oil for injection 10g
[0068] Anhydrous sodium sulfite 0.1g, dibutyl hydroxytoluene 0.5g
[0069] Water for injection to 100ml
[0070] craft
[0071] 1) In the preparation equipment, 4 g of methylprednisolone palmitate is dissolved in 10 g of olive oil for injection;
[0072] 2) Mix 4 g of poloxamer with appropriate amount of water for injection, and add 0.1 g of anhydrous sodium sulfite and 0.5 g of dibutyl hydroxytoluene.
[0073] 3) Add the mixture obtained in step 1) to the mixture obtained in step (2) under stirring conditions, stir and pass through a homogenizer at 40°C, homogenize the solution repeatedly to obtain a uniform solution, add 8g of glucose as a freeze-drying protective agent , to prepare 100ml of liquid medicine, pass through a 0.22μm microporous membrane to sterilize, and pack aseptically under 100 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com