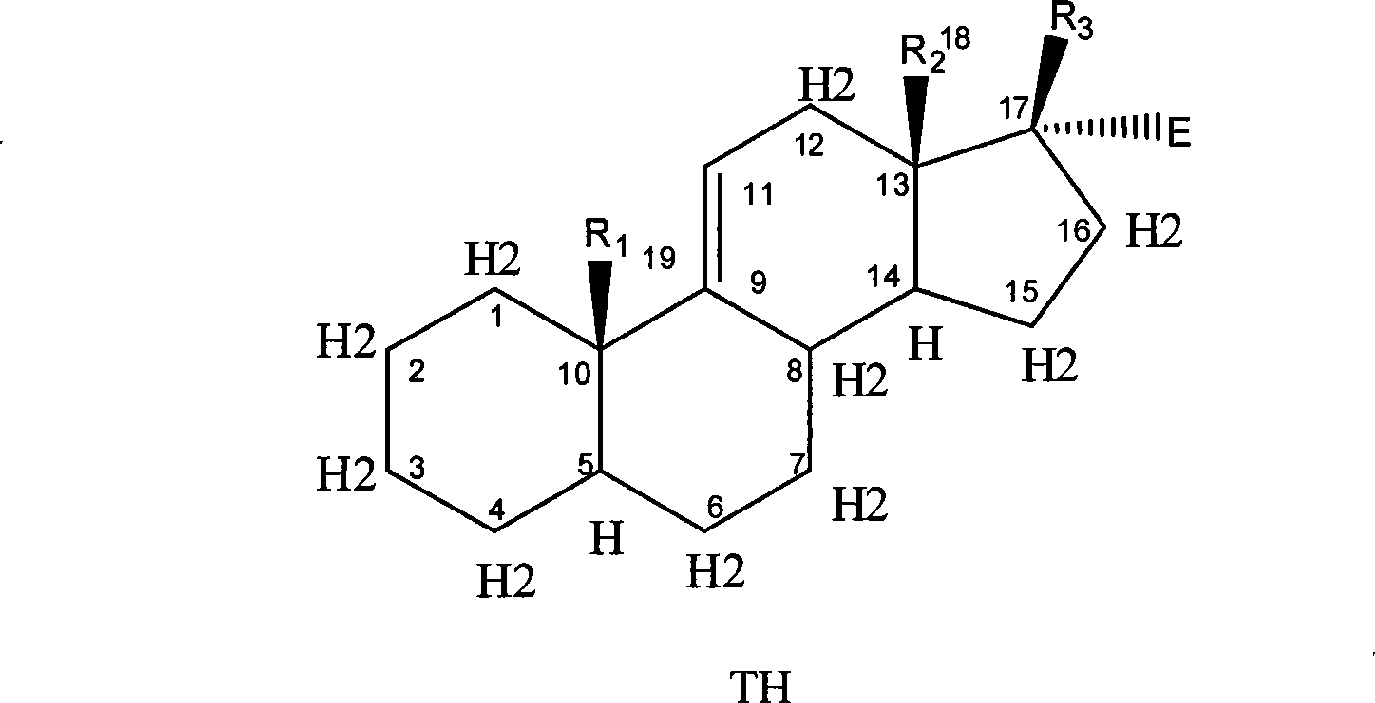
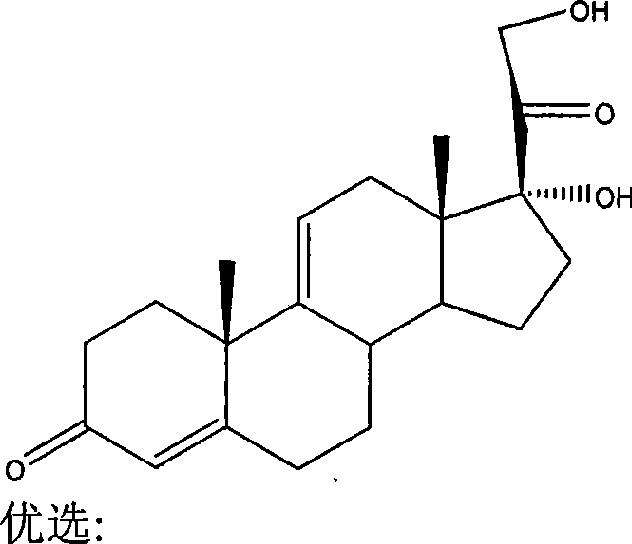
Nitric acid ester medicament for inhibiting angiogenesis
A technology of drugs and compounds, applied in the field of medicine, can solve problems such as ineffective regression of new blood vessels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130] Example 1 Pregna-4,9(11)-diene-3,20-diketone-17,21-dihydroxy-17-(3-nitrooxy)propionate-21-acetate
[0131]
[0132] 1. Pregna-4,9(11)-diene-3,20-diketone-17,21-dihydroxy-17-(3-chloro)propionate-21-acetate
[0133] method 1:
[0134] 40ml of triethylamine, 0.05g of DMAP and 0.015mol of 3-chloropropionyl chloride were stirred in the reaction and cooled to 0 degrees, slowly added 0.01mol of pregna-4,9(11)-diene-3, 20-diketone-17, 21-dihydroxy-21-acetate and keep the temperature at 0-5 degrees. This reaction is an exothermic reaction, so the addition speed and temperature need to be carefully controlled. Keep the temperature of the above reaction solution at 10°C Below, slowly rise to 10-15 degree in 1 hour after adding, and after 15 hours of this temperature insulation, dilute to 50mlPH and be 1-2 the water that temperature is 0 degree, slowly add hydrochloric acid and adjust pH to be 3-5, this The liquid was extracted three times with 90ml of dichloromethane (30mlX3)...
Embodiment 2
[0145] Example 2 Pregna-4,9(11)-diene-3,20-diketone-17,21-dihydroxy-17-(3-nitrooxy)propionate
[0146]
[0147] Dissolve 0.002mol of pregna-4,9(11)-diene-3,20-diketone-17,21-dihydroxy-17-(3-nitrooxy)propionate-21-acetate in methanol and 10ml of chloroform (1:1), under the protection of nitrogen, add dropwise 0.0025mol aqueous sodium carbonate solution saturated at 0°C at 0°C, after stirring for 10 hours, adjust the pH of the reaction system to be neutral with hydrochloric acid, then pressurize and concentrate to remove Chloroform, the solution was diluted in 40ml of ice water, filtered, and dried to obtain the crude product of the title compound. The crude product was subjected to column chromatography and eluted with methanol and chloroform (1:4) as the mobile phase. The main compound was concentrated under reduced pressure, washed into methanol for recrystallization, and 0.001 mol of the title compound was obtained.
[0148] Elemental analysis calculated value (%): C, 62...
Embodiment 3
[0153] Example 3 Pregna-4,9(11)-diene-3,20-diketone-17,21-dihydroxy-21-(3-methyl-4-nitrooxy)-2-thiophenecarboxylic acid ester
[0154]
[0155] With pregna-4,9(11)-diene-3,20-diketone-17,21-dihydroxyl as raw material, according to the method of Example 1 and 4-bromo-3-methyl-2-thienyl Acid chloride, AgNO 3 The reaction affords the title compound.
[0156] 1. Pregna-4,9(11)-diene-3,20-diketone-17,21-dihydroxy-21-(3-methyl-4-bromo)-2-thiophenecarboxylate
[0157] method 1:
[0158] Stir 40ml of triethylamine and 0.015mol of 4-bromo-3-methyl-2-thiophenoyl chloride in the reaction chamber and cool down to 0 degrees, slowly add 0.01mol of pregna-4,9(11)-bis En-3,20-diketone-17,21-dihydroxy and keep the temperature at 0-5 degrees. This reaction is an exothermic reaction, so the addition speed and temperature need to be carefully controlled. Keep the temperature of the above reaction solution below 10°C After the addition, slowly rise to 10-15 degrees within 1 hour, and after...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com