Capecitabine sustained and controlled release oral formulation and preparation method thereof
A technology of capecitabine and controlled-release preparations, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, block delivery, etc., which can solve the problems of large doses of preparations, troublesome, low drug content, etc., and achieve safety Guaranteed, stable blood drug concentration, and improved compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Membrane-controlled sustained-release pellets, the weight percentage ratio is as follows:
[0032] Capecitabine 80%
[0033] Microcrystalline Cellulose 10%
[0034] Sodium Carboxymethyl Cellulose 2%
[0035] Ethylcellulose 8%
[0036] Preparation process: adopt the method known in the pharmaceutical industry, mix the main drug and the filler microcrystalline cellulose, add the aqueous solution prepared by adding the binder carboxymethyl cellulose sodium, prepare the soft material, sieve, granulate, and prepare the drug-containing pellets, and then with ethylcellulose aqueous dispersion ( ) coating liquid for coating.
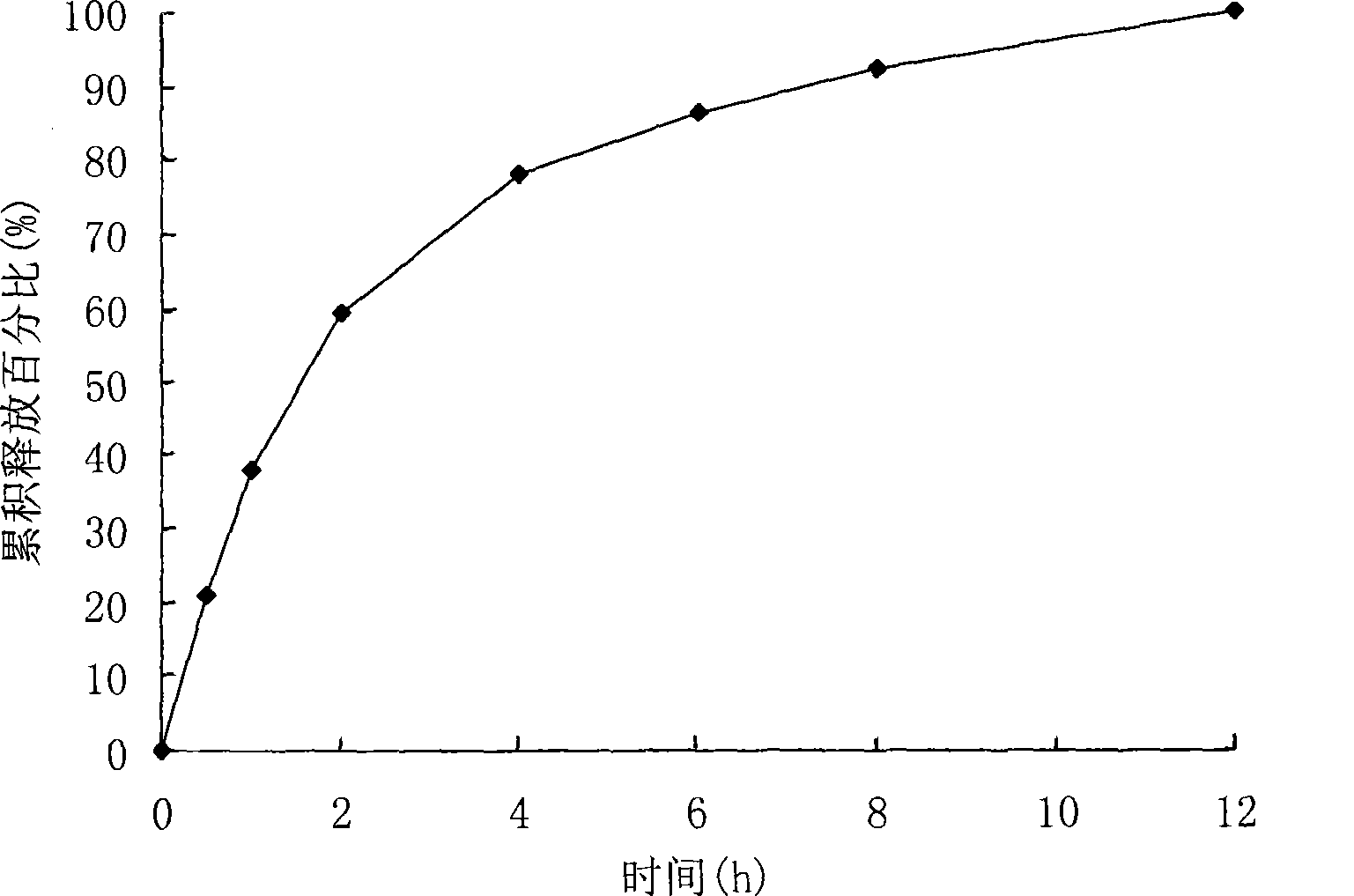
[0037] The membrane-controlled slow-release pellets can be continuously released for 12 hours under the conditions of 37°C, 900ml degassed distilled water, and 100 revolutions per minute by the basket method. The release profile of this formulation is shown in figure 1 .
Embodiment 2
[0039] The sustained and controlled release tablet of hydrophilic gel matrix material, its weight percentage proportioning is as follows:
[0040] Capecitabine 51%
[0041] Polyvinylpyrrolidone 6%
[0042] Lactose 8%
[0043] Hypromellose 34%
[0045] Preparation process: Using a known method in the pharmaceutical industry, uniformly mix the main drug with the binder polyvinylpyrrolidone, the filler lactose and the slow-release material hydroxypropyl methylcellulose, prepare the soft material with absolute ethanol, sieve, and granulate , dried, granulated, added with lubricant magnesium stearate, mixed evenly, and compressed into tablets.
[0046]The sustained and controlled release tablet of the hydrophilic gel skeleton material can be continuously released for 16 hours at 37° C., 900 ml of degassed distilled water, and paddle method at 100 revolutions per minute.
Embodiment 3
[0048] The sustained and controlled release matrix tablet combined with two kinds of wax lipid matrix materials, its weight percentage ratio is as follows:
[0049] Capecitabine 52%
[0050] Carnauba Wax 20%
[0051] Stearic Acid 17%
[0052] Lactose 10%
[0054] Preparation process: using a method known in the pharmaceutical industry, the main drug is mixed with the slow-release material carnauba wax and stearic acid, and the filler lactose is uniformly mixed, granulated with water, dried, granulated, and the lubricant stearic acid is added Magnesium is mixed and pressed into tablets.
[0055] The slow-controlled release matrix tablet combined with two kinds of wax lipid matrix materials can release continuously for 24 hours under the condition of 37°C, 900ml degassed distilled water, paddle method and 100 revolutions per minute.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com