Method for synthesizing alpha-chlorine (2-chlorine) methyl phenyl acetate
A technology for the synthesis of methyl phenylacetate and its method, applied in the field of synthesis of α-chlorophenylacetate methyl ester, can solve the problems of many impurities, difficult to remove, impure products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
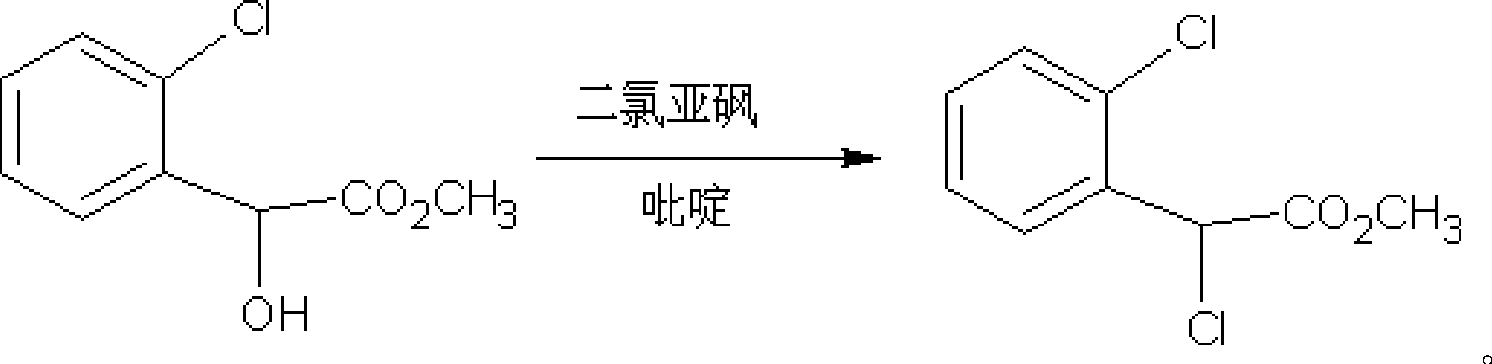
[0022] Embodiment 1 Preparation of methyl o-chloromandelate
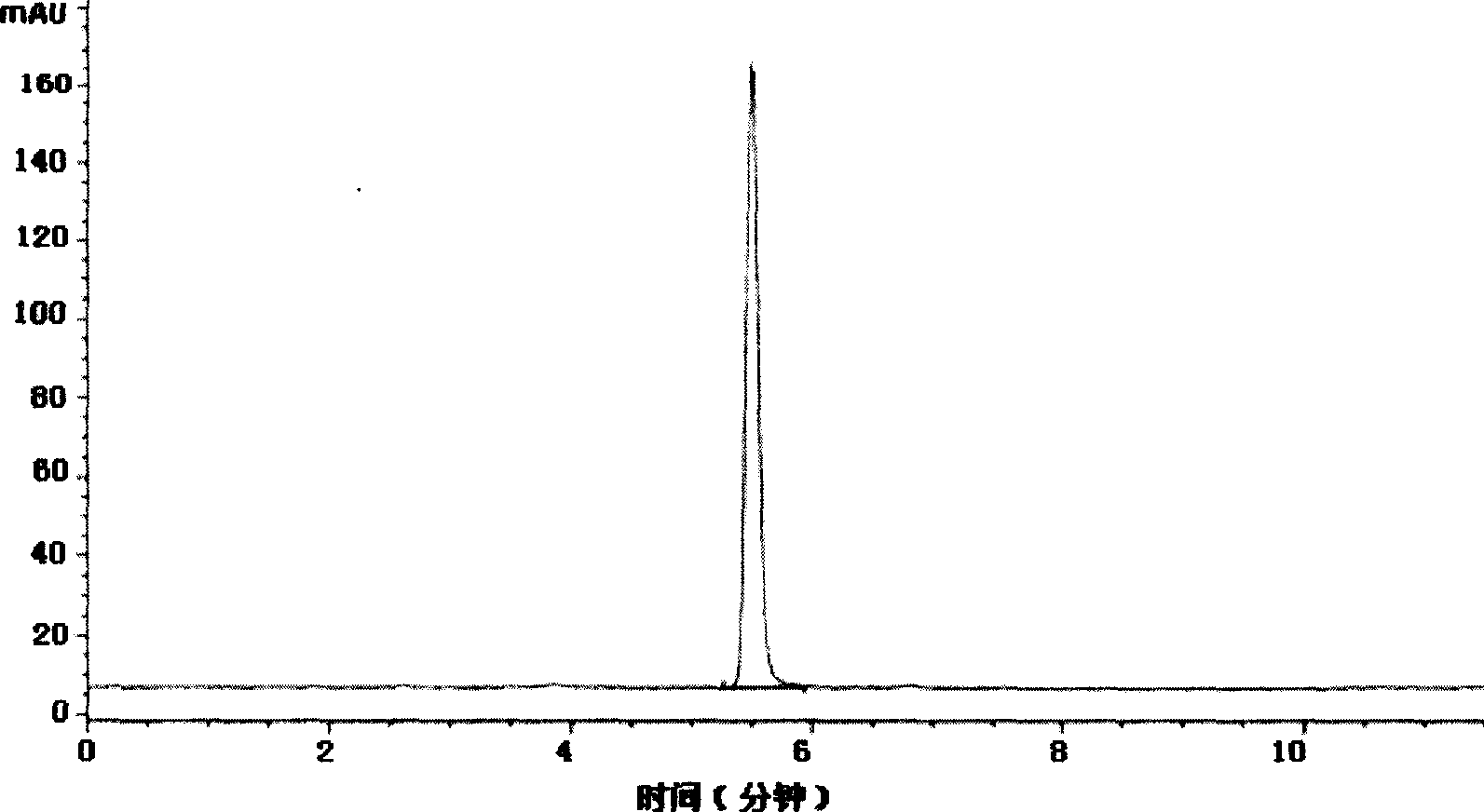
[0023] Measure 10L of methanol into a 30L reaction kettle, add 1000g of o-chloromandelic acid, stir until completely dissolved, slowly add 500ml of concentrated sulfuric acid dropwise, after the dropwise addition is complete, heat up to 80°C for 3 hours, cool to room temperature; use sodium hydroxide solution Adjust the pH to neutral. 5 L of water was added, and 8 L of ethyl acetate was added for extraction. The ethyl acetate layer was washed with saturated sodium bicarbonate, dried, and concentrated to obtain 920 g of methyl o-chloromandelate.
Embodiment 2
[0024] Example 2 Preparation of α-chloro(2-chloro)methyl phenylacetate
[0025] Add 80g of methyl o-chloromandelate and 38.4g of pyridine into the reaction flask, stir, dissolve, and cool to 0°C. Slowly add 50 g of thionyl chloride dropwise. After the dropwise addition was completed, the ice bath was removed, and the temperature of the reaction solution rose slowly to 40°C. The temperature was controlled at 40° C. and stirring was continued for 1 h, and the reaction was detected by HPLC, and the conversion rate was 100%. Filter and wash the filter residue with ethyl acetate. Combine the filtrate and washing liquid, extract with water to remove impurities, add anhydrous sodium sulfate to remove water, filter to remove solid sodium sulfate residue, and evaporate the solvent from the filtrate under reduced pressure to obtain 87 g of methyl α-chloro(2-chloro)phenylacetate, HPLC purity 99.9%, NMR data: 1 H NMR (CDCl 3 ): δ (ppm) = 3.78 (s, 3H), 5.89 (S, 1H), 7.3-7.6 (m, 4H). ...
Embodiment 3
[0026] Example 3 Preparation of α-chloro(2-chloro)methyl phenylacetate
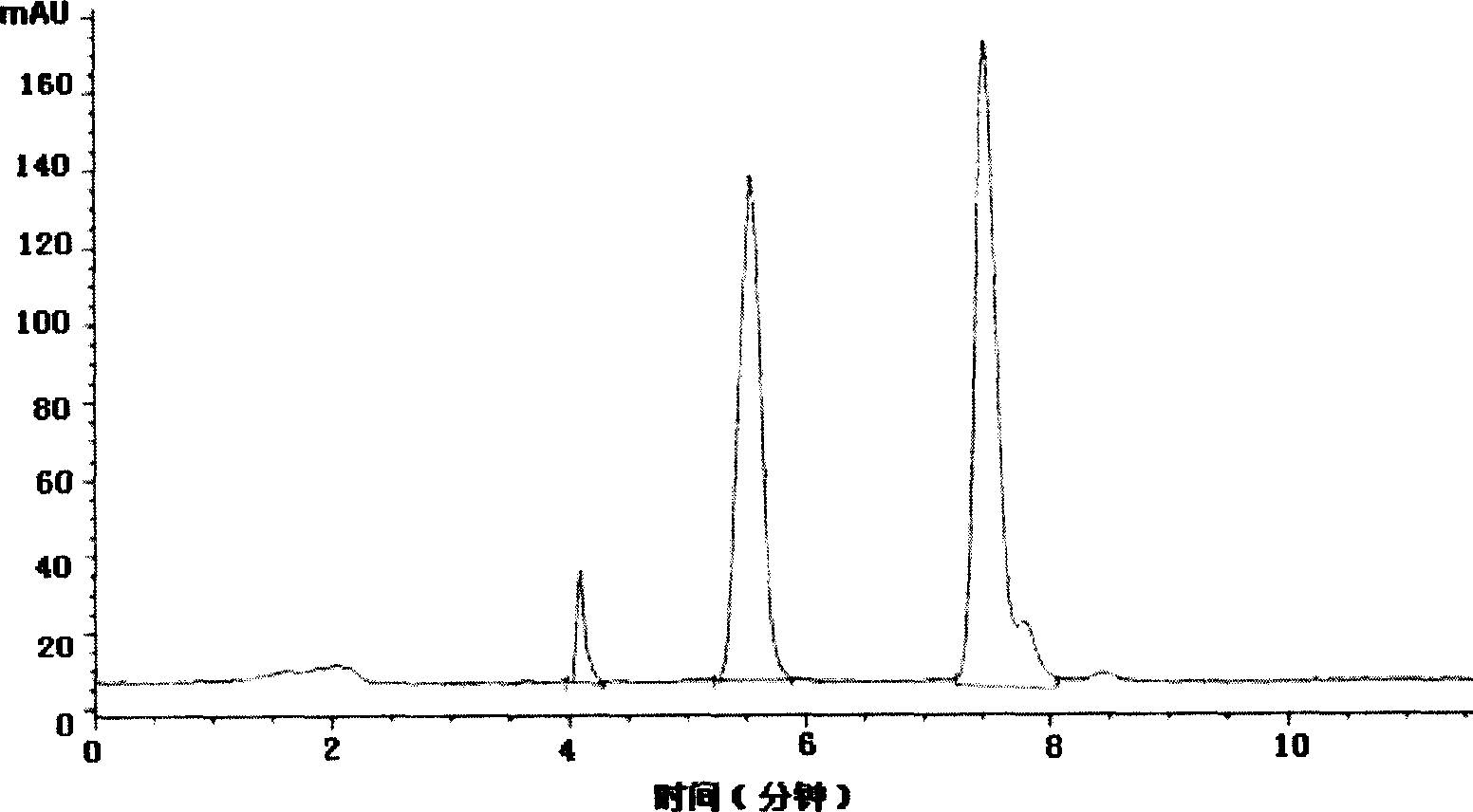
[0027] Add 80g of methyl o-chloromandelate and 38.4g of pyridine into the reaction flask, stir, dissolve, and cool to 0°C. Slowly add 25 g of thionyl chloride dropwise. After the dropwise addition was completed, the ice bath was removed, and the temperature of the reaction solution rose slowly to 40°C. Stirring was continued at 40° C. for 1 h under temperature control, and the reaction was detected by HPLC with a conversion rate of 48%. Filter and wash the filter residue with ethyl acetate. Combine the filtrate and washing liquid, extract with water to remove impurities, add anhydrous sodium sulfate to remove water, filter to remove solid sodium sulfate residue, and evaporate the solvent from the filtrate under reduced pressure to obtain 80 g of crude product, methyl α-chloro(2-chloro)phenylacetate HPLC purity was 40%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com