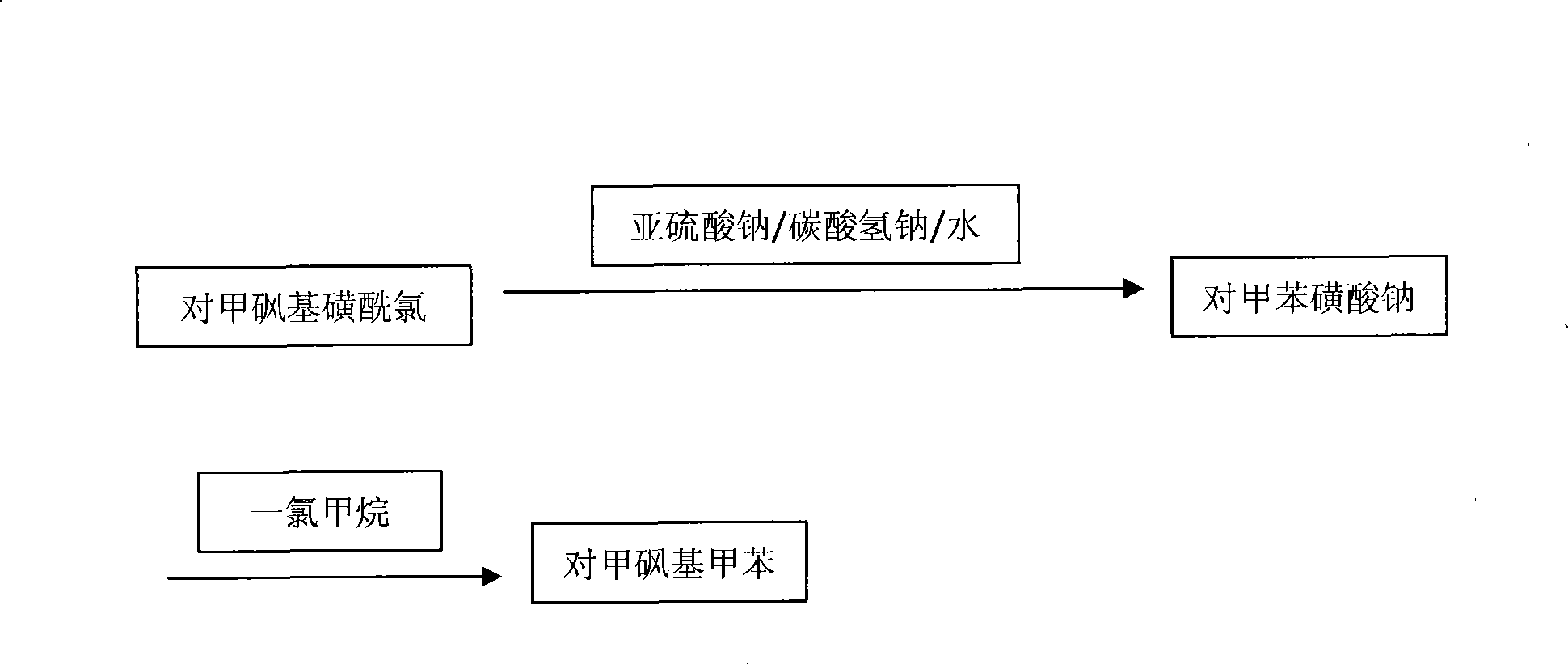
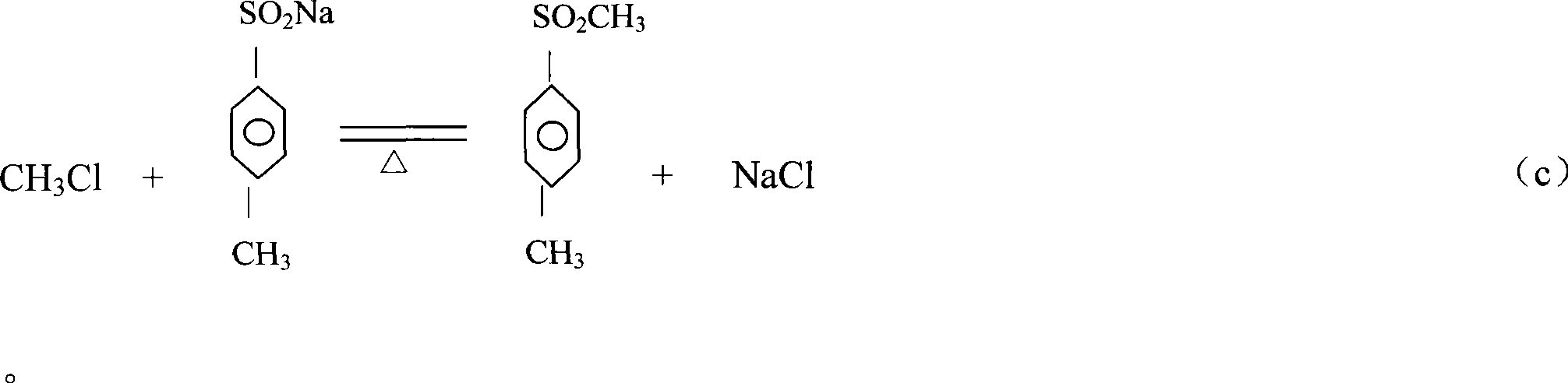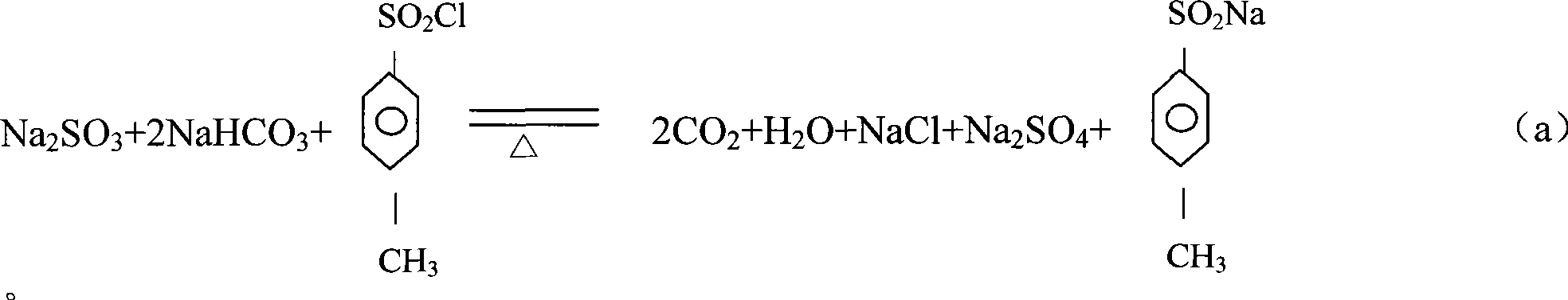Production process of methyl p-tolyl sulfone
A technology of methylsulfonyl toluene and p-methylsulfonyl toluene, which is applied in the field of production technology of p-methylsulfonyl toluene, can solve the problems of high production cost, high toxicity of dimethyl sulfate, long cycle and the like, and achieves reduction of production cost and cycle time. , reduce the risk of environmental pollution, and facilitate the effect of safe production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 1800kg of water, 700kg of p-methylsulfonesulfonyl chloride, 450kg of sodium bicarbonate and 330kg of sodium sulfite to the salt-forming kettle, and carry out a salt-forming reaction under normal pressure, with a reaction temperature of 60°C and a holding time of 4 hours. The carbon dioxide gas produced by the reaction and a small amount of After the reaction is completed, filter at 40-50°C while it is still hot. The mother liquor is pumped into the methylation kettle by vacuum, and the filter residue is treated separately. The mother liquor is further heated to 70°C in the methylation tank at 0.5kg / cm 3 Under pressure, pass 158kg of monochloromethane gas, keep warm and cool to crystallize. When the crystallization mother liquor is cooled to 40°C, it is discharged and filtered, and the filter cake is centrifuged by a centrifuge. The filtered mother liquor and the centrifuged mother liquor are concentrated and distilled. The water vapor is condensed by the condenser a...
Embodiment 2
[0027] Add 1700kg of water, 720kg of p-methylsulfonesulfonyl chloride, 450kg of sodium bicarbonate and 300kg of sodium sulfite to the salt-forming kettle to perform a salt-forming reaction under normal pressure. The reaction temperature is 60°C and the holding time is 3 hours. The carbon dioxide gas and a small amount of water produced by the reaction Empty, after the reaction is completed, filter at 45°C while it is still hot, vacuum pump the mother liquor into the methylation kettle, and process the filter residue separately. The mother liquor is further heated to 85°C in the methylation tank at 0.4kg / cm 3 Under pressure, pass 140kg of monochloromethane gas, keep warm and cool to crystallize. When the crystallization mother liquor is cooled to 40°C, it is discharged and filtered, and the filter cake is centrifuged by a centrifuge. The filtered mother liquor and the centrifuged mother liquor are concentrated and distilled. The water vapor is condensed by the condenser and use...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com