Dibenzyl amine derivatives as cetp inhibitors
A compound, technology of alkyl, applied in the field of dibenzylamine derivatives as cholesteryl ester conversion protein inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
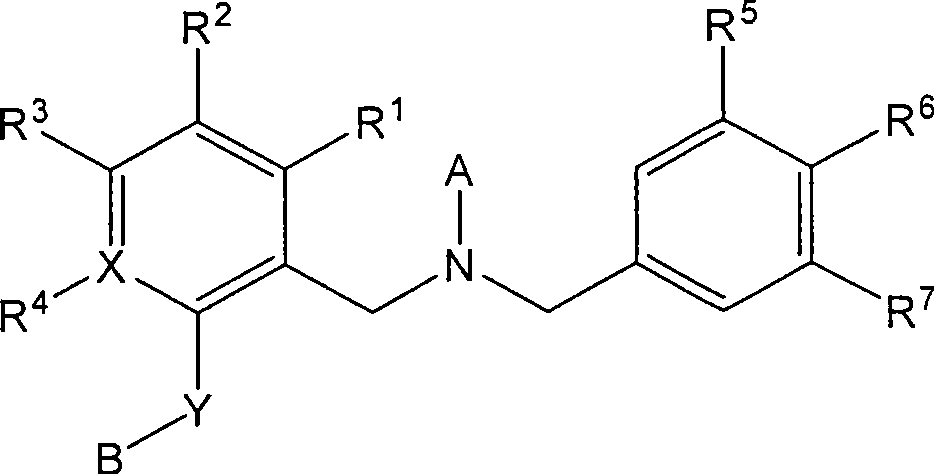
[0364] Example 1: N-[3,5-bis(trifluoromethyl)benzyl]-N-[2-[(dimethylamino)methyl]-5-(trifluoromethyl) Benzyl]-2-methyl-2H-tetrazol-5-amine
[0365]
[0366] Under nitrogen, 2-{[(3,5-bis-trifluoromethyl-benzyl)-(2-methyl-2H-tetrazol-5-yl)-amino]-methyl}-4- To a solution of trifluoromethyl-benzaldehyde (0.025 g, 0.048 mmol) in dichloroethane (5 mL) was added dimethylamine (0.008 g, 0.195 mmol) and tetramethylammonium triacetoxyborohydride (0.038 g, 0.146 mmol). The reaction mixture was stirred at room temperature for 14 hours. LC-MS indicated the formation of the desired product (M+1 = 541.0). The reaction was partitioned between water and dichloromethane. The organic layer was separated and concentrated, then diluted with dimethyl sulfoxide (DMSO), and analyzed in preparative HPLC (Shimadzu, 30x50 C18, basic, 30-95%, 0.1% sodium hydroxide (NaOH), 8 min gradient, 220 UV) Purification above yielded 14.4 mg (54%) of the title compound. 1 H NMR (400MHz, CDCl 3 )δ2.1(s,...
Embodiment 2
[0367] Example 2: N-[3,5-bis(trifluoromethyl)benzyl]-2-methyl-N-[2-(morpholin-4-ylmethyl)-5-(trifluoro Methyl)benzyl]-2H-tetrazol-5-amine
[0368]
[0369] Morpholine (8.5 mg, 0.0977 mmol) in dichloroethane was used according to the procedure described in Example 1. The reaction and purification as described in Example 1 yielded 16.6 mg (58%) of the title compound. 1 H NMR (400MHz, CDCl 3 )δ 2.4 (s, 2H) 3.4 (s, 2H) 3.6 (s, 2H) 4.2 (s, 2H) 4.7 (s, 2H) 5.0 (s, 2H) 7.4 (s, 1H) 7.4 (dd, 1H) 7.5(dd,1H)7.6(s,2H)7.7(s,1H).MS(ES + ) calculated value: 582.4, found value: 583.0 (M+1).
Embodiment 3
[0370] Example 3: N-[3,5-bis(trifluoromethyl)benzyl]-2-methyl-N-[2-(1-morpholin-4-yl-propyl)-5-(tri Fluoromethyl)benzyl]-2H-tetrazol-5-amine
[0371]
[0372] To a solution of benzotriazole (25.6 mg, 0.215 mmol) in ethanol (2 mL) was added morpholine (18.7 mg, 0.215 mmol), and the reaction mixture was stirred at room temperature for 10 minutes. Add 2-{[(3,5-bis-trifluoromethyl-benzyl)-(2-methyl-2H-tetrazol-5-yl)-amino]-methyl}-4-trifluoromethyl - A solution of benzaldehyde (100 mg, 0.195 mmol) in ethanol (2 mL), the reaction mixture was further stirred for 16 hours. MS indicates the formation of MH from the imine intermediate + = 581.4. Ethanol was removed by evaporation, and the residue was dissolved in toluene (3 mL). Ethylmagnesium bromide (0.19 mL, 0.586 mmol, 3M in ether) was added and the reaction mixture was heated at 50°C for 2 hours. LC-MS indicated the formation of the desired product. The reaction mixture was diluted with ethyl acetate, then washed with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| absorption coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com