Catalyst for hydrogen production from catalytic pyrolysis of natural gas and preparation method thereof
A catalytic cracking and catalyst technology, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc., can solve the problems of easy deactivation, affecting catalyst stability, and anti-coking Poor ability and other problems, to achieve the effect of improving stability, inactive surface properties, and strong anti-coking ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
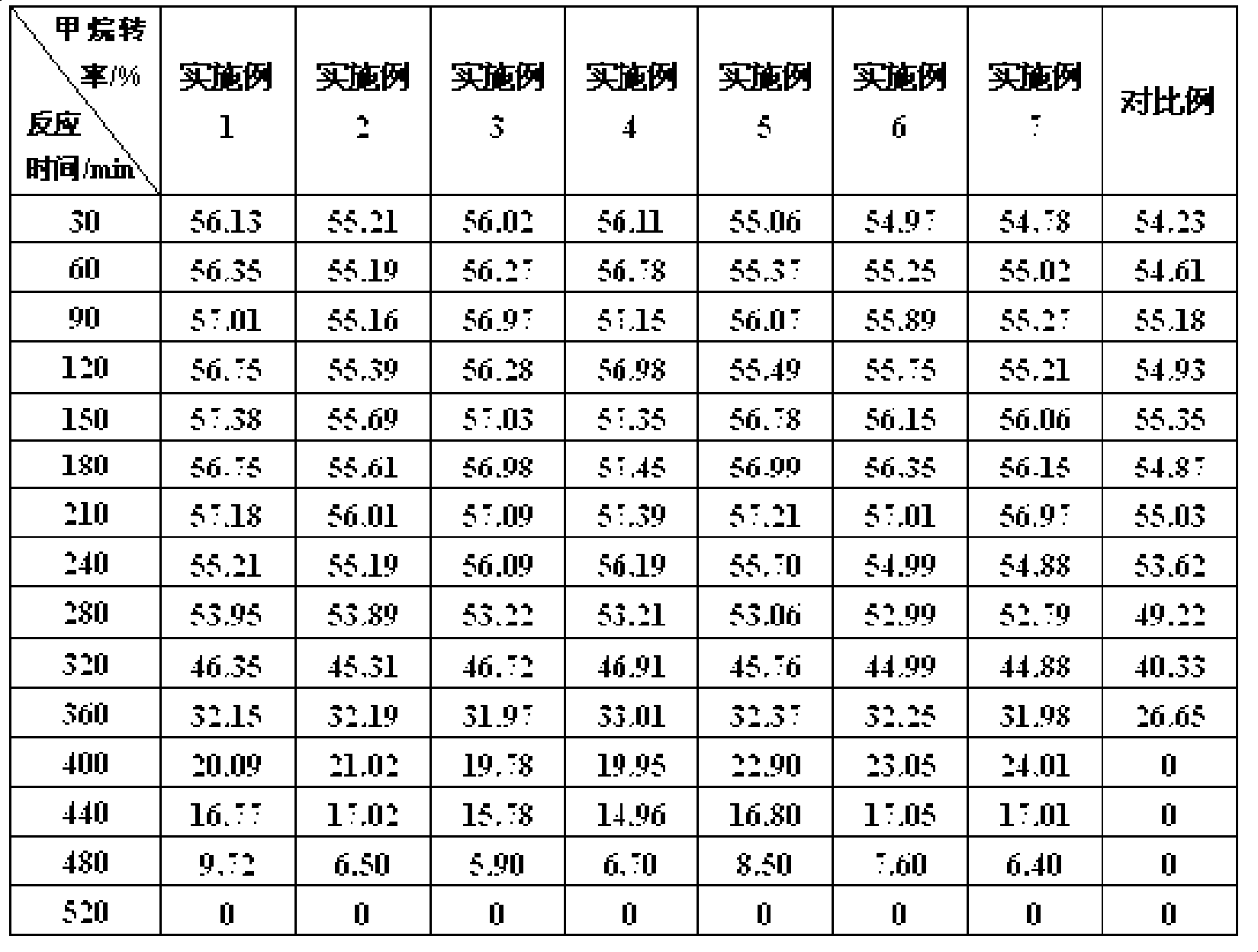
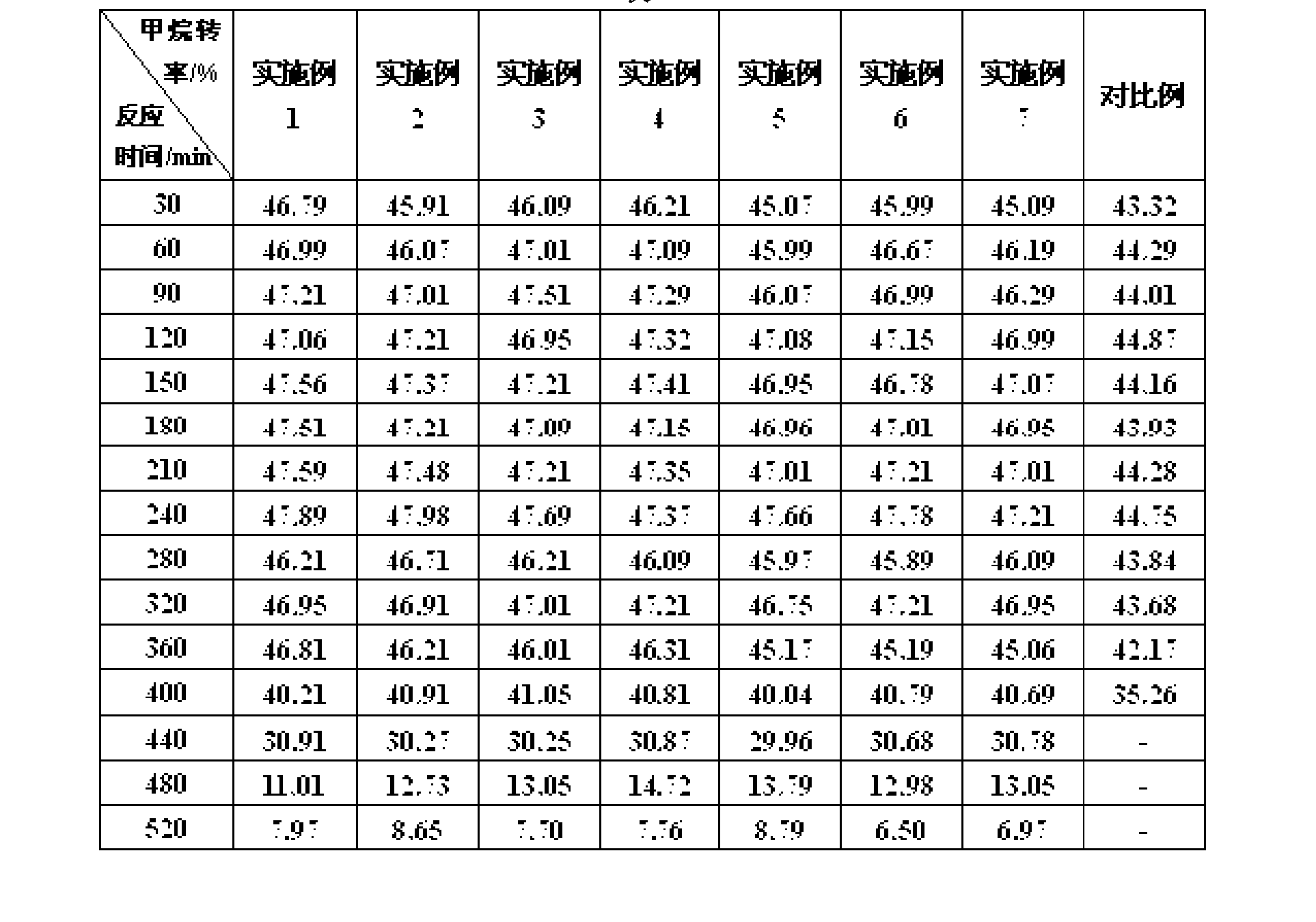
Image
Examples
Embodiment 1
[0024] The parts by weight of each component in the catalyst are: Ni element 10, SiO 2 60, CNFs 45; prepare nickel nitrate and SiO according to the above ratio 2 , The amount of CNFs added.
[0025] ①Soak CNFs in 20% nitric acid by mass, boil and reflux for 2 hours, filter, wash the obtained CNFs to pH 6, dry at 80°C for 24 hours, and then dry at 800°C Roasting for 1h;
[0026] ② SiO 2 Add the CNFs obtained in step ① into an aqueous solution of nickel nitrate at a temperature of 60°C and stir for 4 hours, then add ammonia water to adjust the pH value to 6, continue to stir for 6 hours, and then let it stand for 8 hours. Dry it for 24 hours to get it.
Embodiment 2
[0028] The parts by weight of each component in the catalyst are: Ni element 50, TiO 2 5, CNFs 30; prepare nickel chloride and TiO according to the above ratio 2 , The amount of CNFs added.
[0029] ① Soak CNFs in nitric acid with a mass percentage of 50%, boil and reflux for 1 hour, filter, wash the obtained CNFs to pH 7, dry at 120°C for 2 hours, and then dry at 600°C Roasting for 3h; then repeat the above process 2 times for the obtained CNFs;
[0030] ② TiO 2 Add the CNFs obtained in step ① into an aqueous solution of nickel chloride at 90°C and stir for 1 hour, then add sodium carbonate solution to adjust the pH value to 7, continue stirring for 4 hours, and then let it stand for 2 hours. It is obtained by drying at 90°C for 10 hours.
Embodiment 3
[0032] The parts by weight of each component in the catalyst are: Ni element 30, ZrO 2 70, CNFs 2; prepare nickel acetate, ZrO according to the above ratio 2 , The amount of CNFs added.
[0033] ①Soak CNFs in 70% nitric acid by mass, boil and reflux for 1.5h, filter, wash the obtained CNFs to pH 6.5, dry at 100°C for 5h, then dry at 350°C Lower roasting for 8h; then repeat the above process once for the obtained CNFs;
[0034] ② ZrO 2 Add the CNFs obtained in step ① into an aqueous solution of nickel acetate at a temperature of 70°C and stir continuously for 0.5h, then add sodium hydroxide solution to adjust the pH value to 8, continue stirring for 3h, and then stand for 6h, and the obtained precipitate is filtered and washed , Drying at a temperature of 100 ° C for 8 hours, that is, too.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com