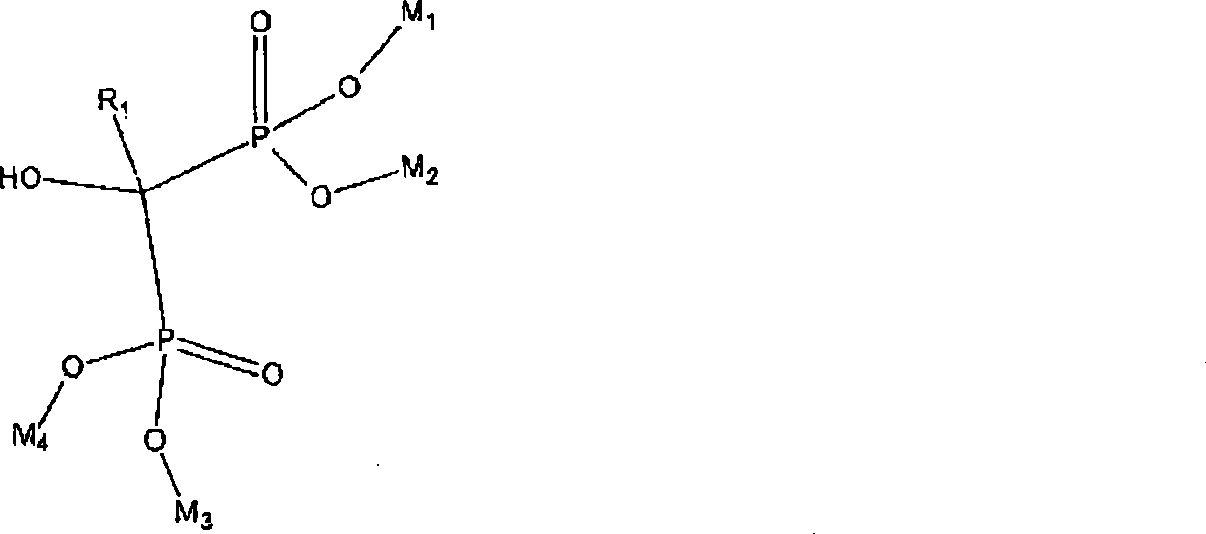
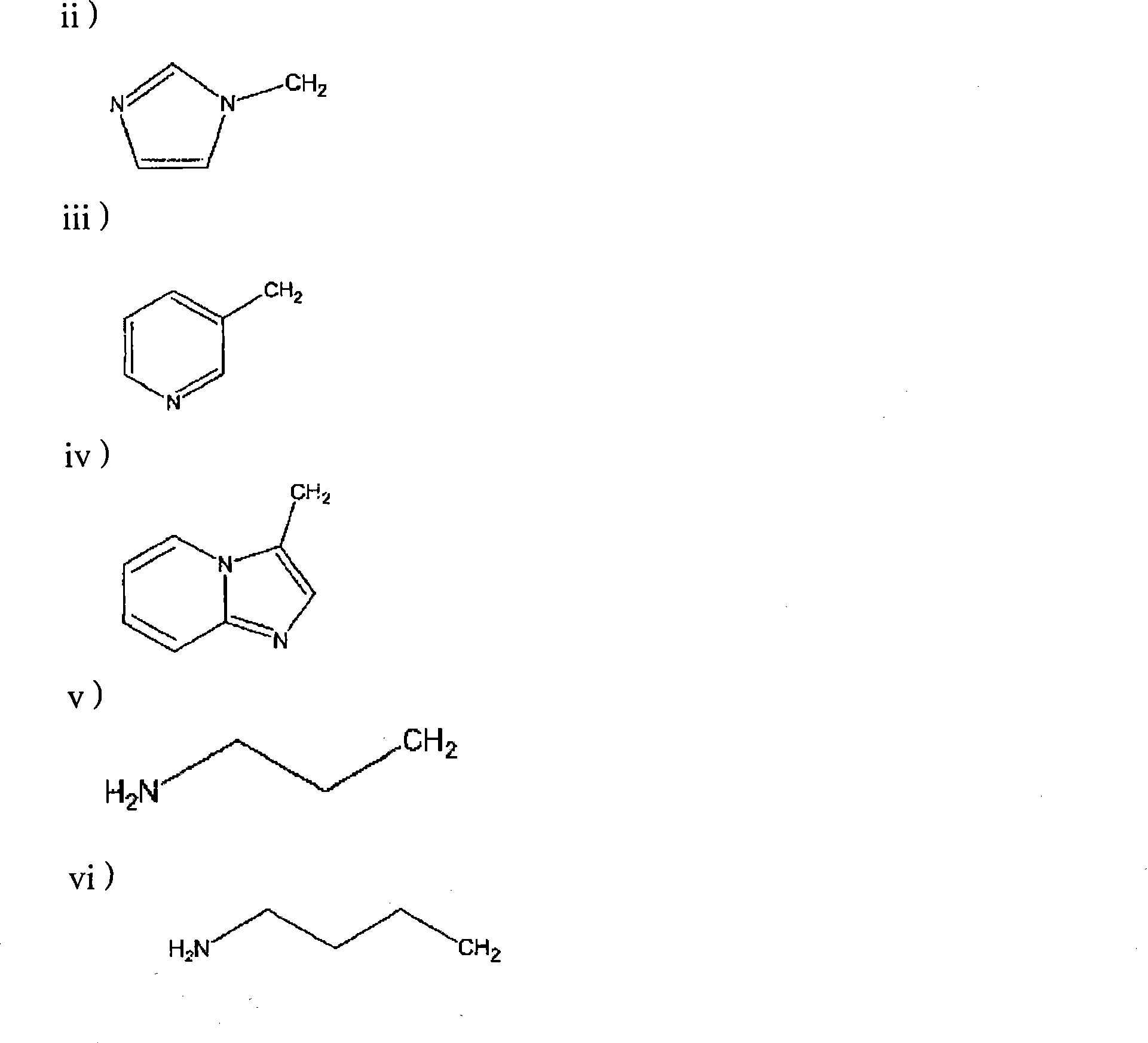
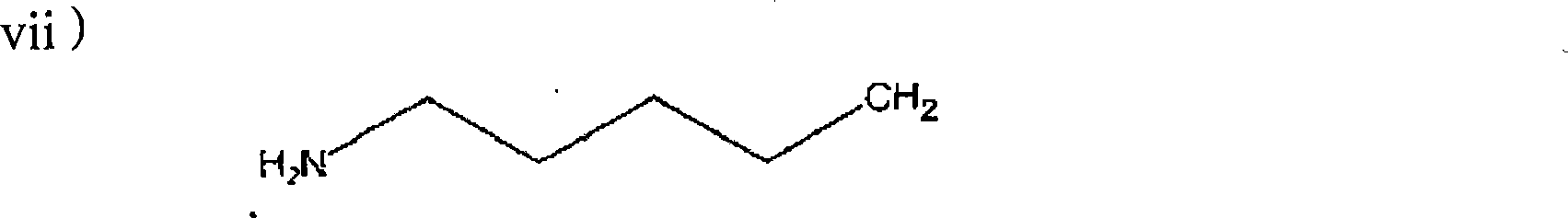
Process for manufacturing bisphosphonic acids
A technology of diphosphonic acid and phosphoric acid, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve problems such as unsatisfactory yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of imidazole acetic acid:
[0033] A 50 L reactor was charged with chloroform (54 kg), imidazole (6.13 kg, 90.04 mol) and tert-butyl chloroacetate (5.48 kg, 36.4 mol). The temperature was raised to 60°C over 2 hours and held at 60°C for an additional 24 hours. The reaction mass was cooled to room temperature. The chloroform phase was washed successively with 4 portions of water (7.2 kg each) to remove the imidazolium salt and excess imidazole.
[0034] Water (15.1 kg) was added and chloroform was removed by distillation using a Dean-Stark trap at a mantle temperature of 60-65°C (the boiling point of the azeotrope is 53°C) to recover the aqueous phase . After removal of the chloroform, the reactor mantle temperature was slowly raised to 115°C, during which time t-butanol and water were co-distilled (the azeotrope boils at 80°C). After removal of the alcohol, the aqueous solution was cooled and drained from the reactor, yielding 17.54 kg of a solution cont...
Embodiment 2
[0036] Separation of imidazole acetic acid:
[0037] Part of the solution in Example 1 (1.13 kg) was rotovaped to obtain a solid slurry (0.38 kg), and acetone (234 g) was added to complete crystallization. The solid was filtered, washed with acetone, and dried with a stream of nitrogen. The evaporator condensate was evaporated again, washed and dried to obtain a second crystalline product; combined with the first crystalline product to obtain imidazole acetic acid (analysis by NMR measurement: 219 g, recovery 91%, purity 98.9 wt%). 1 H NMR (D20): 8.68 (s, 1H); 7.42 (s, 2H); 4.83 (s, 2H); 4.79 (br s, 1H).
Embodiment 3
[0039] Preparation of zoledronic acid:
[0040] A 1.5 liter tank reactor equipped with heating mantle, mechanical stirrer, Dropping funnel, thermocouple and condenser with nitrogen inlet adapter. Phosphorus trichloride (330 g, 2.41 mol) was added slowly to the reaction mass, exothermic and hydrogen chloride was produced. The temperature was allowed to rise to 70°C and the solution was stirred until HCl evolution ceased. The temperature of the reaction mass rose to 85°C and a white solid began to form which floated and stuck to the stirrer shaft. After about 1 hour, stirring was no longer possible and the stirring motor was stopped. The reaction mass was heated at 85°C for 5 hours or more and then cooled to room temperature to form a solid, homogeneous white material.
[0041]Slow addition of water (320ml) to the white mass resulted in an exotherm and formation of HCl. The water slowly dissolves the material in a gradual and uniform manner, eventually freeing the agitator....
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com