Method for preparing 2-nitrine-1, 6-D-anhydro-sugars
A technology of inner ether sugar and 6-D-, which is applied in chemical instruments and methods, dehydrated sugar, sugar derivatives, etc., can solve the problems of environmental hazards for operators and unsuitability for industrialization, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
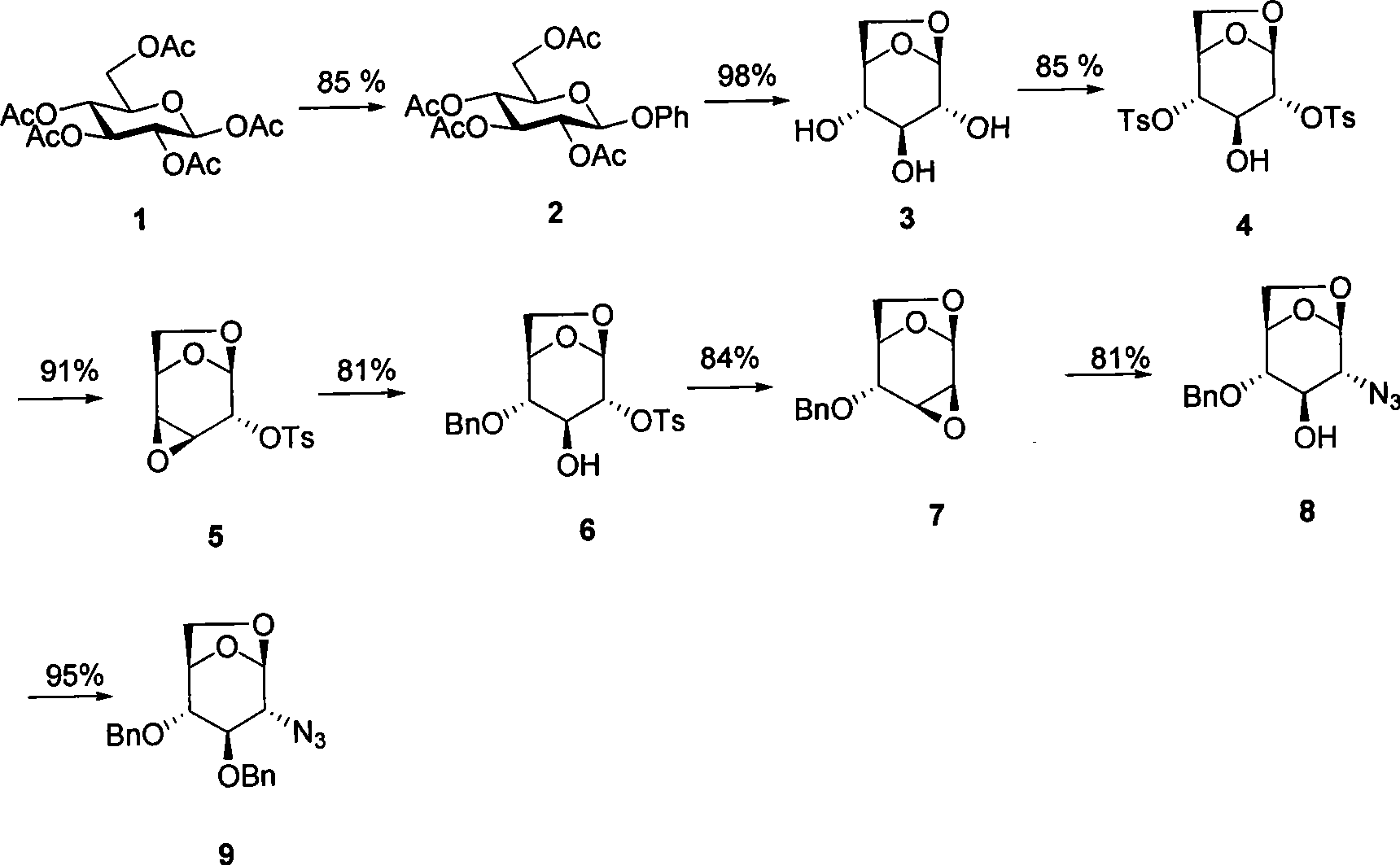
[0021] (4) selective preparation of 3,4-position epoxy compound 5;
[0022] (5) Benzyl alcohol opens epoxy to prepare compound 6;
[0023] (6) Preparation of 2,3-position epoxy compound 7 under alkaline conditions;
[0024] (7) Azide opens epoxy to prepare compound 8;
[0025] (8) Benzyl protects the hydroxyl group to prepare the final product 9;
[0026] Wherein in step 1: fully acetylated D-glucose or L-glucose 1 and phenol are catalyzed by Lewis acid to prepare 2,3,4,6-tetraacetyl-phenyl glycoside; phenol or pentachloro Phenol, the dosage is 1 to 3 times of 1 (molar ratio); Lewis acid can be selected from halogenated boron, halogenated titanium, and halogenated tin, and fed in the calculated amount; the solvent is selected from C 1 -C 6 Monohalogen or polyhalogen alkanes, acetonitrile, dioxane, DMF, can be a single solvent or a mixed solvent, and the composition ratio is not specified; the reaction temperature is -10°C~100°C, the best reaction temperature is 0°C~ 30°C;...
Embodiment
[0035] Preparation of D-2,3,4,6-O-tetraacetylphenoside 2
[0036] Peracetyl-D-glucose 1 (390 g, 1 mol) was dissolved in 2 liters of dichloromethane, followed by adding phenol (141 g, 1.5 mol), triethylamine (51 g, 0.5 mol), under nitrogen protection, trifluoro Boronium ether (355 g, 2.5 mol) was slowly added dropwise to the above solution. After the dropwise addition was completed, the mixture was stirred at room temperature for 3 hours. The completion of the reaction was tracked and monitored by TLC. The reaction was quenched by adding 2 liters of saturated sodium bicarbonate solution, separated, and the aqueous phase was extracted with 2 liters of dichloromethane. The combined organic phases were dried, filtered and concentrated. A light yellow syrup 2 (360 g, 0.85 mol) was obtained with a yield of 85%.
[0037] Preparation of 2,3,4-trihydroxy-1,6-D-lactyl ether sugar 3
[0038]Light yellow syrup 2 (360 g, 0.85 mol) was placed in a 3L single-necked bottle, 2L of 1.3M KOH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com