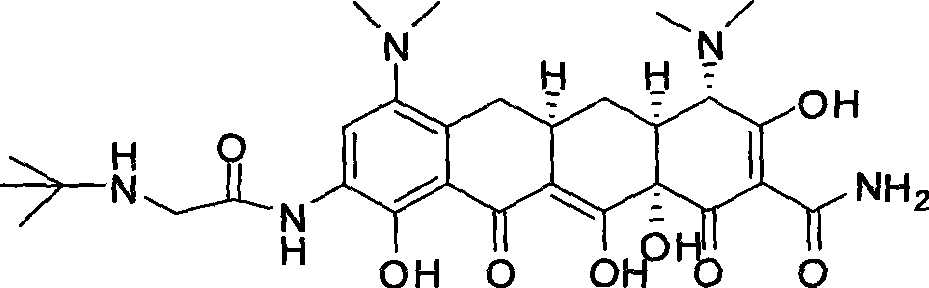
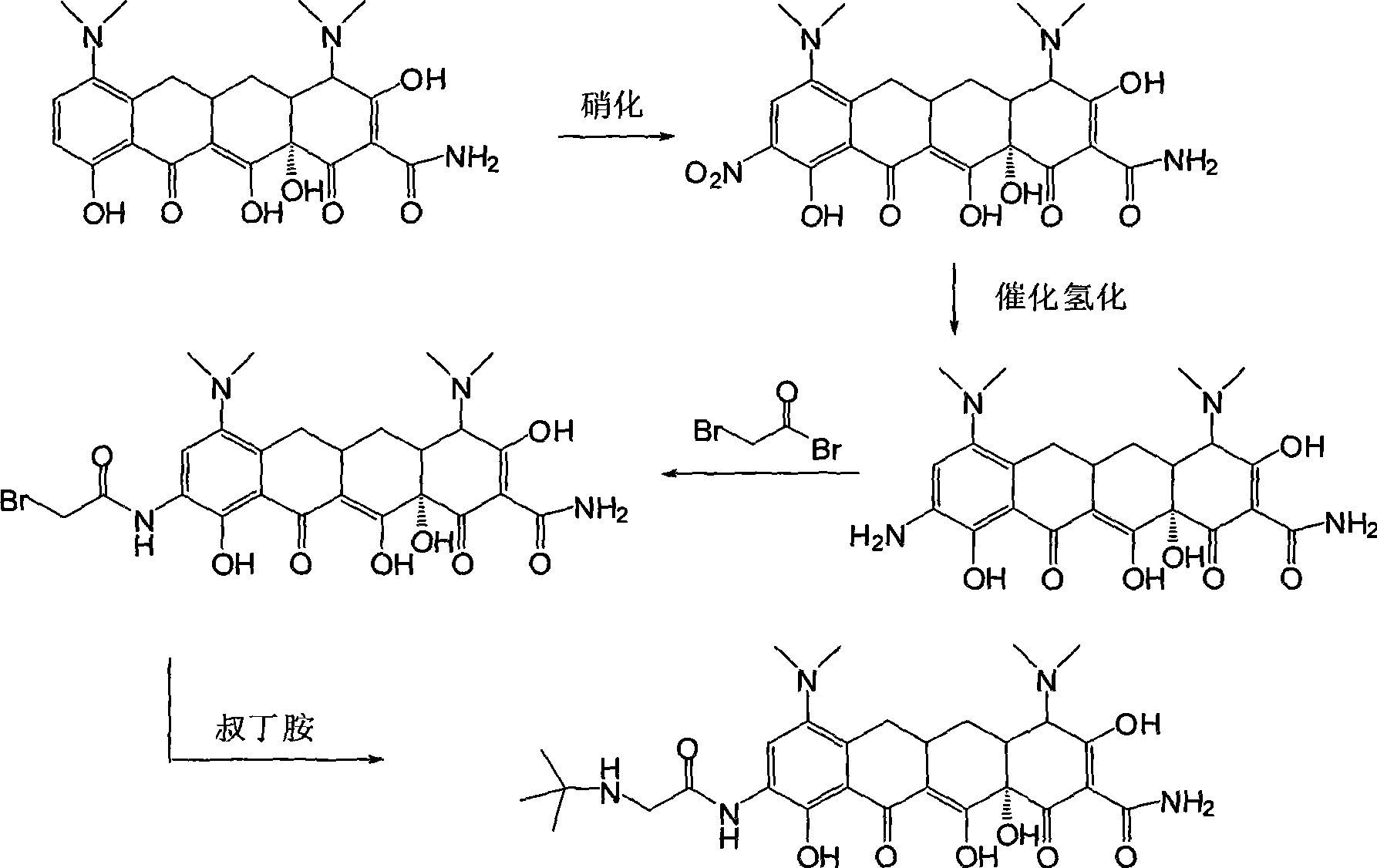
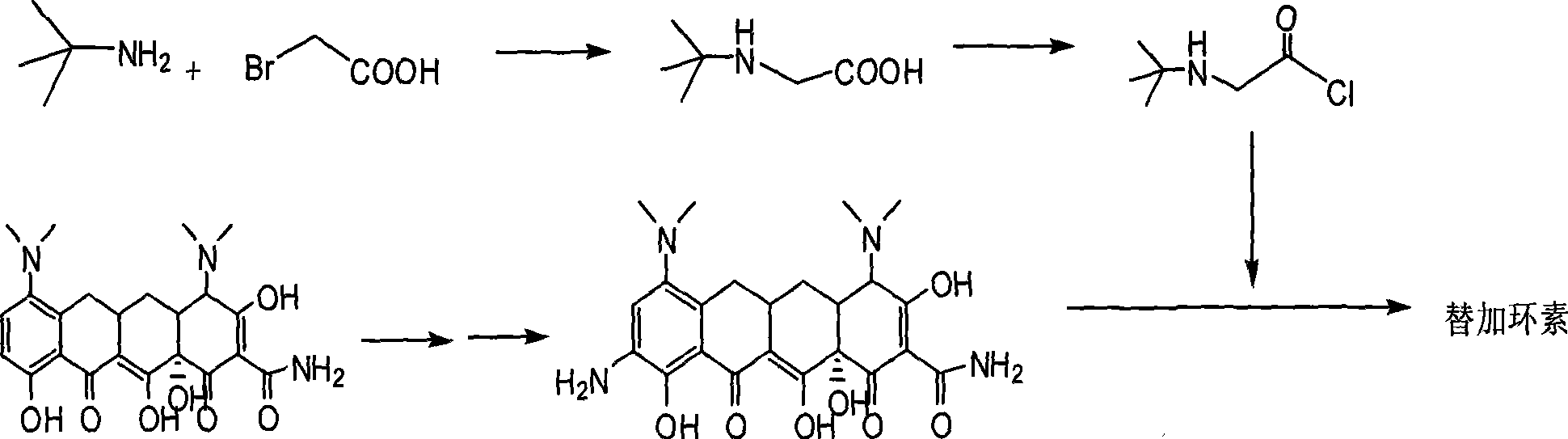
Synthetic method of tigecycline
A synthesis method and technology of tigecycline, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of high cost and low yield, and achieve easy control of conditions, simple operation, and wide application foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 , the preparation of sancycline (I)
[0035] According to the method of the U.S. Patent (US3160661) of the people such as Jerry Robert Daniel McCormick, 6-demeclotetracycline is carried out catalytic hydrogenation, obtains product (I), specifically as follows:
[0036] Take 4.6g of 6-demeclotetracycline, dissolve it in a mixture of 100mL of water and dimethylformamide DMF (1:1), adjust the pH value to 1.8 with concentrated hydrochloric acid, add 3.0g of 5% Pd-C, and then Add 0.5mL of perchloric acid dropwise, place in a hydrogen reactor, pass through hydrogen, and stir for 2h at a pressure of 1.5atm.
[0037] The reaction solution was filtered to remove the catalyst, the filtrate was adjusted to pH 3.0 with ammonia water, extracted with 100 mL of n-butanol, the organic phase was washed once with the same volume of saturated NaCl, then dried with anhydrous sodium sulfate, and spin-dried under reduced pressure to obtain 3.0 g Yellow powder (I).
[0038] Mass ...
Embodiment 2
[0039] Example 2 , 7, the preparation of 9-dinitroshancycline (II)
[0040] According to the method of the U.S. Patent (US3397230) of Robert Winterbottom et al., product (I) is carried out nitration reaction, obtains product (II), specifically as follows:
[0041] Take 60mL of concentrated sulfuric acid and stir in an ice-water bath to lower the temperature to about 0°C. Take 4.0 g of compound (I) and slowly add it to concentrated sulfuric acid, controlling the temperature not to exceed 20°C. After the addition is complete, stir until the temperature drops below 10°C. Weigh 4.0gKNO 3 , added to the reaction solution in three batches, and stirred at this temperature for 15 min after addition, and thin layer chromatography TLC showed that the reaction was complete. The reaction solution was slowly poured into 600 mL of frozen anhydrous diethyl ether, a precipitate was precipitated, left to stand, and filtered with suction to obtain 5.5 g of light red solid (II).
[0042] T...
Embodiment 3
[0043] Example 3 , the preparation of 7-nitro-9-aminocycline (III)
[0044] According to Nagaraj R.Ayyangar, Uttam R.Kalkote et al's method (Bull.Chem.Soc.Jpn., 1983,56,3159-3164), the product (II) is selectively reduced to obtain the product (III), specifically as follows:
[0045] Take 2.0 g of compound (II), add 25 mL of 60% hydrazine hydrate, and stir to dissolve. Stirring at room temperature for 6h, TLC showed that the reaction was complete. Under an ice-water bath, the pH value of the reaction solution was adjusted to about 5 with 3N HCl, and the same volume of n-butanol was added for extraction. The organic phase was separated, washed twice with the same volume of water, dried over anhydrous sodium sulfate, and spin-dried under reduced pressure to obtain 1.5 g of reddish-brown solid (III).
[0046] The obtained compound (III) was subjected to mass spectrometry (ESI, Q-Tofmicro YA019), MS: 475 (M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com