Long-acting composite contraception microspheres and preparation method thereof
A technology of microspheres and compound prescriptions is applied in the field of long-acting compound contraceptive microspheres and their preparation, and can solve the problems of large side effects, complicated use methods of long-acting contraceptive implants, affecting liver and kidney functions, and the like.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Preparation of gestodene / ethinyl estradiol microspheres
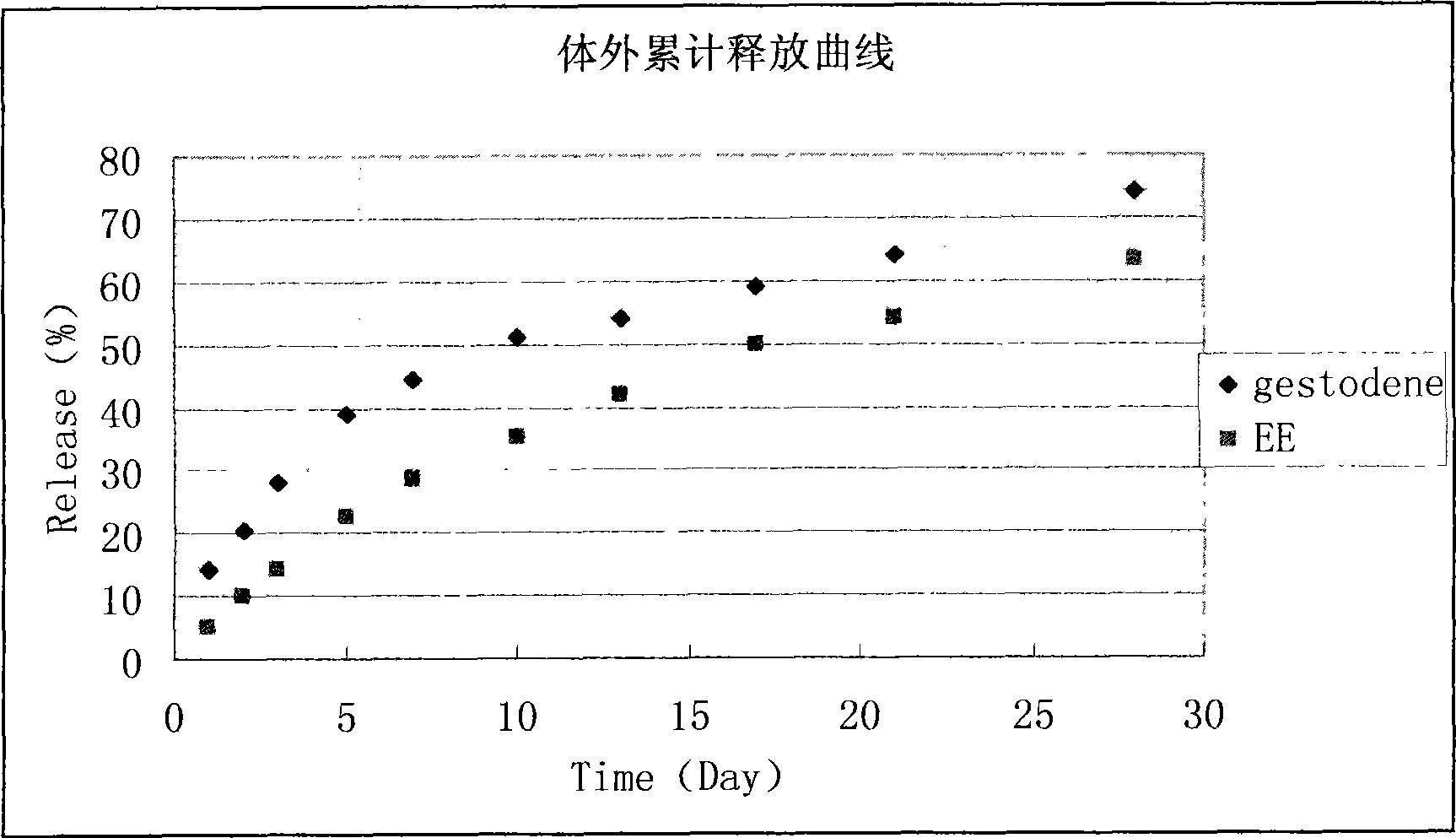
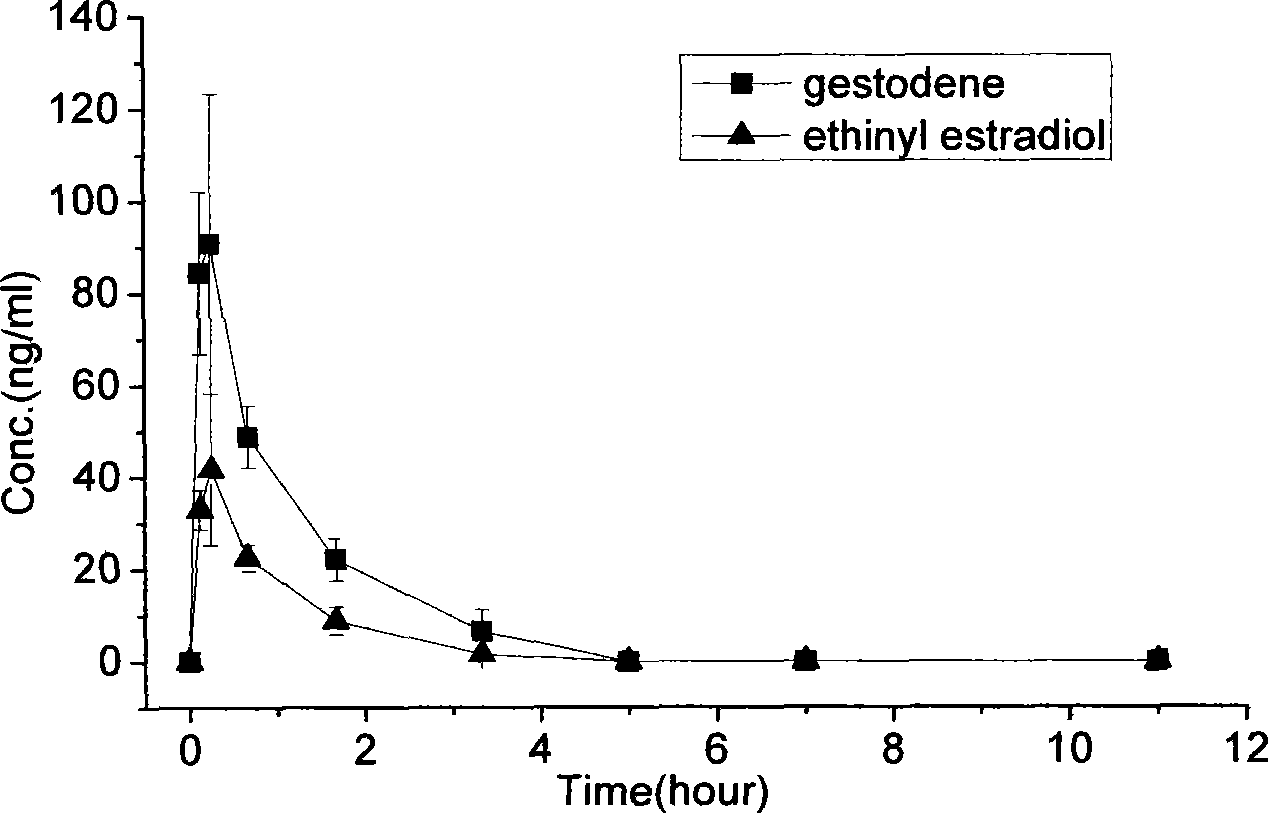
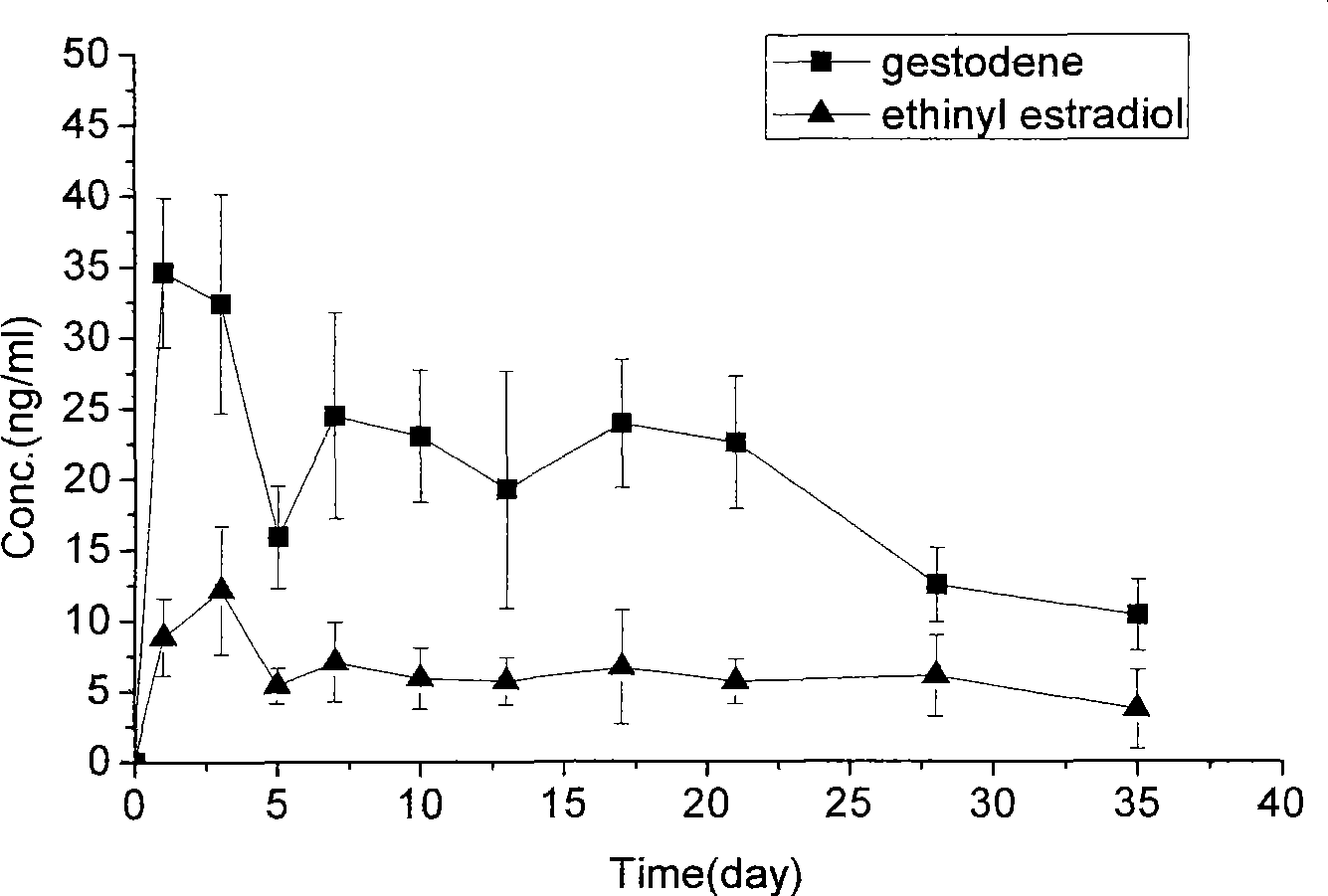
[0059] At room temperature, weigh out 250mg of polylactic acid-glycolic acid (50:50, Mw=10000), 5mg of gestodene and 2mg of ethinyl estradiol, and dissolve them with 2ml of dichloromethane as an organic solvent. The organic solution was quickly added to 60ml of 1% PVA solution, emulsified in an electric stirrer (1200rpm) for 10 minutes, and then poured into 160ml of 1% PVA solution, evaporated at 25°C for about 6 hours, and left to stand to separate the solidified PLGA microspheres , And washed with distilled water and freeze-dried to obtain, the diameter of the microspheres is 40-80 microns.
[0060] The measurement results of the drug encapsulation efficiency of microspheres are as follows: the encapsulation efficiency of gestodene and ethinyl estradiol are 77.61% and 69.38%, respectively.
Embodiment 2
[0061] Example 2 Preparation of gestodene / estradiol microspheres
[0062] At room temperature, weigh out 250mg of polylactic acid (Mw=20000), 5mg of gestodene and 2mg of estradiol, dissolve in 3ml of ethyl acetate as an organic solvent, and quickly add the organic solution containing polylactic acid and drugs to 100ml In 0.2% PVA solution, emulsify in an electric stirrer (2000rpm) for 2 minutes, then pour it into 150ml 1% PVA solution, evaporate at 25°C for about 6 hours, stand still to separate and solidify PLGA microspheres, and wash with re-distilled water It is obtained after freeze-drying, and the diameter of the microspheres is 20-60 microns.
[0063] The experimental results showed that the encapsulation rates of gestodene and estradiol were 86.32% and 74.86%, respectively.
Embodiment 3
[0064] Example 3 Preparation of gestodene / ethinyl estradiol microspheres
[0065] At room temperature, weigh 250mg polylactide-glycolide (75:25, Mw=10000), 5mg gestodene and 2mg ethinyl estradiol, and dissolve them with 5ml dichloromethane as an organic solvent to dissolve the PLGA and Add the organic medicine dropwise to 200ml 0.2% sodium carboxymethyl cellulose solution, emulsify in an electric mixer (1000rpm) for 5 minutes, reduce the stirring speed to 300rpm, evaporate at 25°C for about 6 hours, and stand still to separate the solidified PLGA The microspheres are washed with re-distilled water and then freeze-dried to obtain the microspheres with a diameter of 60-120 microns.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com