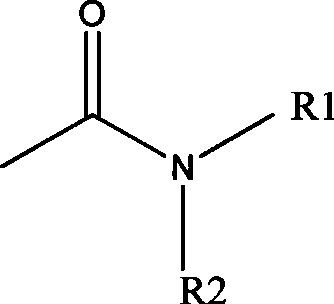
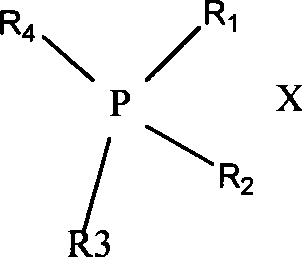
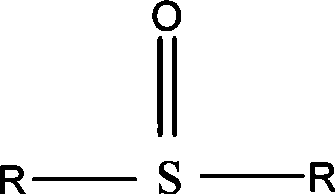Method for preparing 2-chloro-4-fluorobenzene nitrile
A technology of fluorobenzonitrile and nitrobenzonitrile, which is applied in the field of preparing 2-chloro-4-fluorobenzonitrile, can solve the problems of long synthesis steps, environmental pollution, and low yield, and achieve no pollution, easy-to-obtain raw materials, The effect of high reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In a three-necked flask containing 120 g of dimethylacetamide and 58 g of potassium fluoride, add 183 g of 2-chloro-4-nitrobenzonitrile and 2.4 g of catalyst tetraphenyl bromide under stirring, and react at 100-110 °C for 8 ~10h. The solvent dimethylacetamide was distilled off under reduced pressure, the residue was extracted with cyclohexane, and the composition of the extract was analyzed by GC / MS. The extract was rectified under reduced pressure, and 133 g of fractions at 100-120°C / 10Pa were collected and passed through GC. / MS mass spectrometry, m / z: 155.56, proved to be Molecular ion characteristic fragments are measured with a gas chromatograph (GC-2014, purchased from Shimadzu company), the content of 2-chloro-4-fluorobenzonitrile in the fraction is 99.2538%, and the content of 2-chloro-4-fluorobenzonitrile product The yield was 85.26%.
Embodiment 2
[0024] In a three-necked flask containing 714g of dimethyl sulfoxide and 174g of potassium fluoride, add 183g of 2-chloro-4-nitrobenzonitrile and 18g of catalyst tetraethylphosphorous chloride under stirring, and react at 130-150°C for 5 ~8h. The solvent dimethyl sulfoxide was distilled off under reduced pressure, the residue was extracted with cyclohexane, and the composition of the extract was analyzed by GC / MS. The extract was rectified under reduced pressure, and 126 g of fractions at 100-120°C / 10Pa were collected and passed through GC. / MS mass spectrometry, m / z: 155.56, proved to be Molecular ion characteristic fragments are measured with a gas chromatograph (GC-2014, purchased from Shimadzu company), and the content of 2-chloro-4-fluorobenzonitrile in the fraction is 99.3687%, and the content of 2-chloro-4-fluorobenzonitrile product is The yield was 80.77%.
Embodiment 3
[0026] In a three-neck flask filled with 500g of dibutylacetamide and 87g of potassium fluoride, add 330g of 2-chloro-4-nitrobenzonitrile and 4g of catalyst tetraphenylbromophosphorus under stirring, and react at 130°C for 5 to 8 hours . The solvent dibutylacetamide was distilled off under reduced pressure, the residue was extracted with cyclohexane, and the composition of the extract was analyzed by GC / MS. The extract was rectified under reduced pressure, and 228 g of fractions at 100-120°C / 10Pa were collected and passed through GC. / MS mass spectrometry, m / z: 155.56, proved to be Molecular ion characteristic fragments are measured with a gas chromatograph (GC-2014, purchased from Shimadzu company), and the content of 2-chloro-4-fluorobenzonitrile in the fraction is 99.0327%, and the content of 2-chloro-4-fluorobenzonitrile product The yield was 81.05%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com