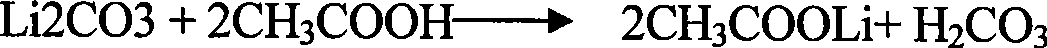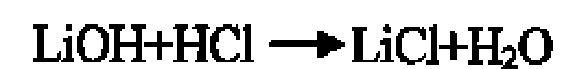Method for preparing high purity lithium fluoride
A high-purity lithium fluoride and lithium fluoride technology, applied in the direction of lithium halide, etc., can solve the problems of reducing LiF production cost, insufficient pretreatment, and high content of transition metal impurity elements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1, a kind of method for preparing high-purity lithium fluoride has the following steps:
[0029] (1) The preparation of lithium hexafluorophosphate by solvent method is usually based on excess LiF and PCl 5 As a raw material, lithium hexafluorophosphate is directly synthesized by metathesis reaction, and the reaction is as follows:
[0030]
[0031] In order to ensure the completeness of the reaction, an excess of 0.1-2 mol of lithium fluoride is usually required in the actual preparation. After the reaction is completed and the obtained lithium hexafluorophosphate is separated and purified, a large amount of mixed by-products of lithium fluoride and lithium chloride will be obtained at the same time.
[0032] (2) drop the mixture of lithium fluoride and lithium chloride into deionized water, stir the solution to fully dissolve the mixture,
[0033] And made into a slightly acidic aqueous solution with a pH value of 0.1 to 7;
[0034] (3) In the state of...
Embodiment 2
[0036] Embodiment 2, a kind of method for preparing high-purity lithium fluoride has the following steps:
[0037] (1) Lithium chloride is obtained after acidifying lithium hydroxide by hydrochloric acid.
[0038]
[0039] (2) drop lithium chloride into deionized water, stir the solution to fully dissolve the mixture, and make a slightly acidic aqueous solution with a pH value of 0.1 to 7;
[0040] (3) In the state of stirring, quickly drop the solution of saturated ammonium bifluoride (no ratio and no temperature requirement) in the aqueous solution described in the above steps to obtain lithium fluoride precipitation; continue to stir for 0.5-1h, and stand still for 1-2h;
[0041] (4) Step 4 is the same as step (4) of Embodiment 1.
Embodiment 3
[0042] Embodiment 3, a kind of method for preparing high-purity lithium fluoride has the following steps:
[0043](1) Lithium acetate is obtained after lithium carbonate is acidified by acetic acid:
[0044]
[0045] (2) Lithium acetate is dropped into deionized water, the solution is stirred to fully dissolve the mixture, and it is made into an acidic aqueous solution with a pH value of 0.1 to 7;
[0046] (3) In the state of stirring, quickly drop saturated ammonia and hydrogen fluoride two elemental solutions (no ratio and no temperature requirement) into the aqueous solution described in the above steps to obtain lithium fluoride precipitation; continue stirring for 0.5-1h, Stand still for 1-2h;
[0047] Step 4 is the same as step (4) of Embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com