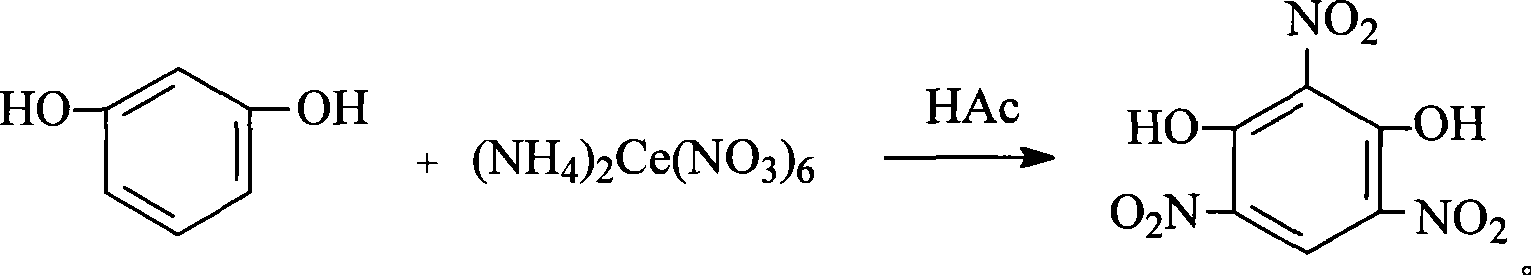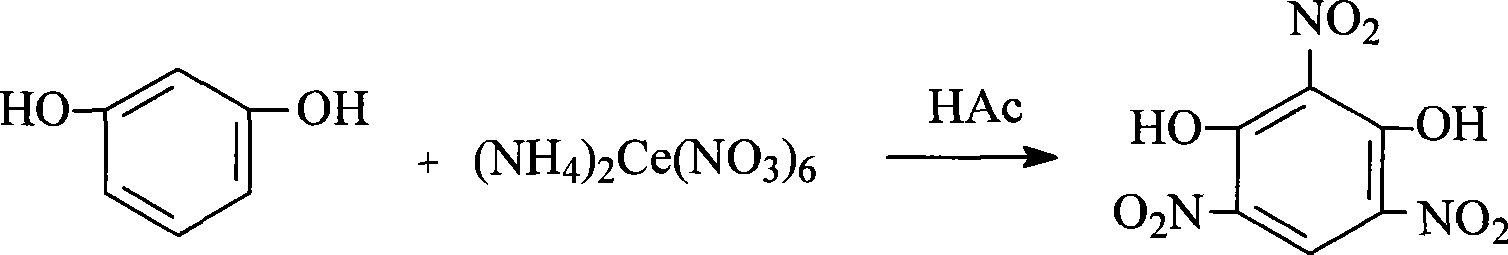Preparation of 2,4,6-trinitroresorcinol
A technology of trinitroresorcinol and resorcinol, which is applied in 2 fields, can solve the problems of long production cycle, large amount of waste acid waste water, and large amount of acid used, and achieves reduced production costs, simple operation, and high reaction rate. short time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Add 0.1101g (1mmol) of resorcinol and 1mL of 50% acetic acid to a 50mL Erlenmeyer flask. Dissolve 0.548g (1mmol) of cerium ammonium nitrate in 4mL of water to obtain an aqueous solution of cerium ammonium nitrate. Under magnetic stirring, add cerium ammonium nitrate dropwise. The aqueous solution was heated to 30°C and reacted for 0.8h. The reactants were completely converted into products (TLC tracking, ethyl acetate: petroleum ether = 2:1), and the stirring was stopped; the product was poured into ice water, and a brownish-red solid precipitated; Suction filtration, washing the precipitate with distilled water twice to obtain 2,4,6-trinitroresorcinol. Weighing: 0.18g, yield: 73.5%, measured melting point: 178°C-179.5°C, IR: v max (KBr tablet, cm -1 ), 3131, 1671, 1506, 1400, 850.6; UV: (anhydrous methanol) λ max : 252.3; MS: m / z(M-H) + : 245.7; 1 HNMR: (DMSO-d 6 , Ppm) δ: 6.31 (s, 2H), 9.16 (s, 1H).
Embodiment 2
[0013] In a 50mL Erlenmeyer flask, add 0.5505g (5mmol) resorcinol and 5mL 25% acetic acid, 3.562g (6.5mmol) cerium ammonium nitrate dissolved in 20mL of water to obtain cerium ammonium nitrate aqueous solution, under magnetic stirring, add cerium nitrate dropwise Aqueous ammonium solution, heated to 45℃, reacted for 1.2h, the reactant was completely converted into product (TLC tracking, ethyl acetate: petroleum ether = 2:1), stop stirring; pour the product into ice water, a brownish-red solid precipitated ; Suction filtration, distilled water washing precipitation 3 times to obtain 2,4,6-trinitroresorcinol. Weighing: 0.882g, yield: 72%, measured melting point: 178.5℃-179.6℃, IR: v max (KBr tablet, cm -1 ), 3130, 1670, 1504, 1400, 850.6; UV: (anhydrous methanol) λ max : 252.5; MS: m / z(M-H) + : 245.7; 1 HNMR: (DMSO-d 6 , Ppm) δ: 6.30 (s, 2H), 9.19 (s, 1H).
Embodiment 3
[0015] Add 66.061g (600mmol) of resorcinol and 60mL of 5% acetic acid in a 1500mL round bottom flask, and dissolve 526.08g (960mmol) of cerium ammonium nitrate in 550mL of water to obtain an aqueous solution of cerium ammonium nitrate. Under magnetic stirring, add cerium nitrate dropwise. Aqueous ammonium solution, heated to 60℃, reacted for 1.6h, the reactant was completely converted into product (TLC tracking, ethyl acetate: petroleum ether = 2:1), stop stirring; pour the product into ice water, a brownish-red solid precipitated ; Suction filtration, distilled water washing precipitation 3 times to obtain 2,4,6-trinitroresorcinol. Weighing: 120.69g, yield: 82.1%, measured melting point: 178.1℃-179.4℃, IR: v max (KBr tablet, cm -1 ), 3132, 1671, 1505, 1401, 851.2; UV: (anhydrous methanol) λ max : 253.1; MS: m / z(M-H) + : 245.6; 1 HNMR: (DMSO-d 6 , Ppm) δ: 6.29 (s, 2H), 9.18 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com