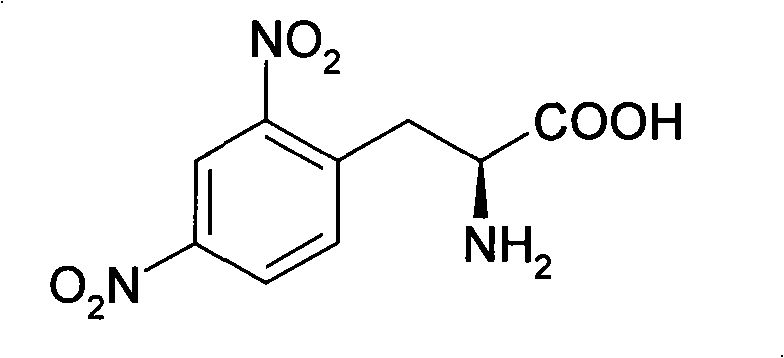
Process for synthesizing L-2,4-dinitrophenyl alanine
A technology of dinitrophenylalanine and nitrophenylalanine, which is applied in the fields of chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., and can solve difficulties in unsuitable for large-scale production, side reactions, and separation of mixtures etc. to achieve the effects of low production cost, mild reaction conditions, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1, a kind of synthetic method of L-2,4-dinitrophenylalanine, carries out following steps successively:
[0018] 1) Add 60g (1.0mol) of urea to 100mL of 63% concentrated nitric acid (1.0mol) at room temperature (25°C). After complete addition, react for 1h and cool in an ice-water bath (0°C) to obtain 121.0g of urea nitrate , yield 98%.
[0019] 2), at 10°C, dissolve 21.0 g of L-4-nitrophenylalanine (0.10 mol) in 50 mL of concentrated sulfuric acid (98% w), then add 14.7 g of urea nitrate prepared in step 1) ( 0.12mol), the reaction was continued for 20h at this temperature. After adjusting the pH of the obtained reaction solution to 7.0 with ammonia water, the obtained solution was dehydrated, and then the obtained solid was recrystallized with 500 mL of methanol, and the yellow solid precipitated from the mother liquor was L-2,4-dinitrobenzene Alanine, a total of 19.7g (yield 77.3%).
[0020] The reaction conditions in Example 1 were changed to obtain Exa...
Embodiment 5
[0023] Embodiment 5, a kind of synthetic method of L-2,4-dinitrophenylalanine, carries out following steps successively:
[0024] 1), at room temperature, 123g (1.0mol) of urea nitrate was added to 200mL of concentrated sulfuric acid (98%w). After the urea nitrate was completely dissolved, the reaction was continued for 1h, and the resulting reaction solution was poured into 500g of ice water to precipitate White solid nitrourea 100g, yield 95.2%.
[0025] 2), at 10°C, dissolve 21.0 g of L-4-nitrophenylalanine (0.10 mol) in 50 mL of concentrated sulfuric acid (98% w), and then add 12.6 g of nitrourea prepared in step 1) (0.12mol), the temperature continued to react for 20h. After the obtained reaction solution was adjusted to pH 7.0 with sodium hydroxide, the obtained solution was dehydrated, and then the obtained solid was recrystallized with 500 mL ethanol to obtain L-2, 23.5 g of 4-dinitrophenylalanine ( Yield 92.2%).
[0026] The reaction conditions in Example 5 were ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com