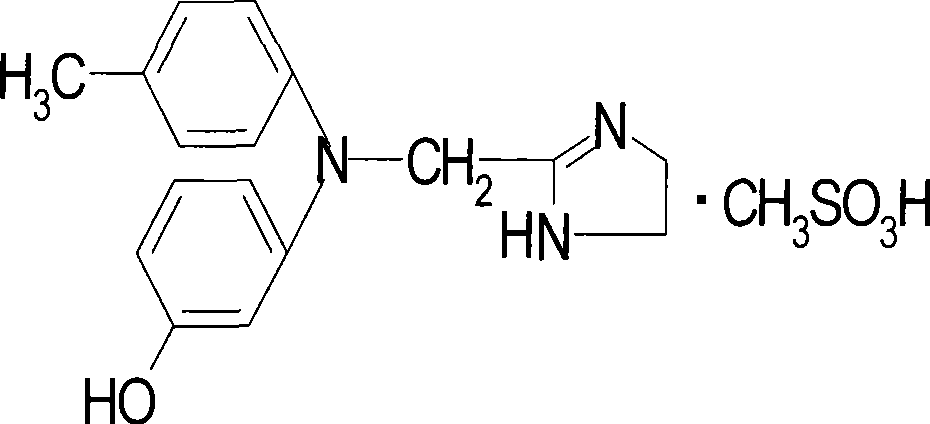
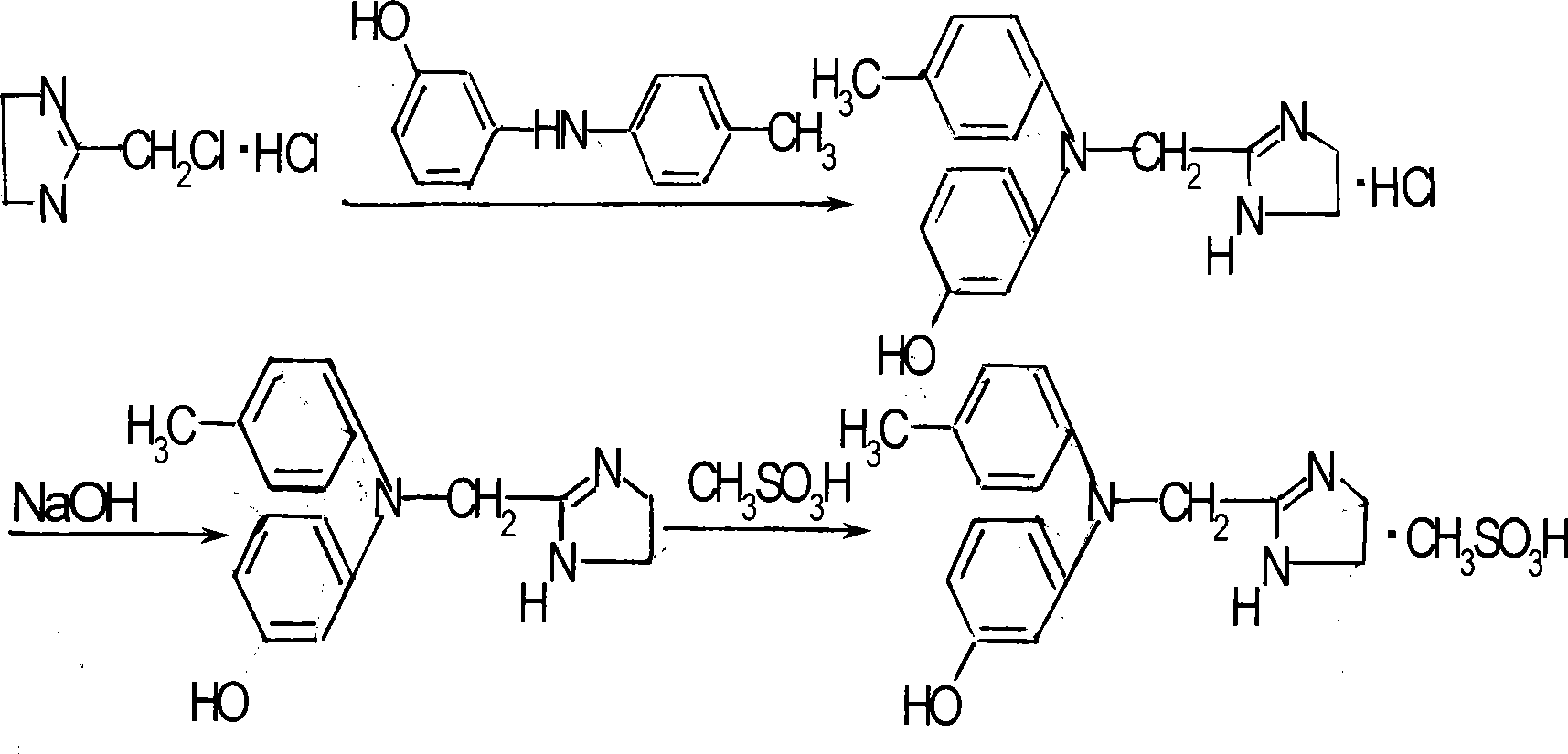
Method for synthesizing phentolamine mesylate
The technology of phentolamine mesylate and phentolamine hydrochloride is applied in the field of synthesis of phentolamine mesylate, which can solve the problems of large stimulation to the human body, high boiling point, complicated process and the like, so as to reduce toxicity and reaction temperature, reducing the formation of impurities, removing irritating effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of Phentolamine Hydrochloride
[0031] Add 8g (0.04mol) of 3-hydroxy-4-methyldiphenylamine, 6.15g (0.04mol) of chloromethylimidazoline hydrochloride, and 64ml of toluene into the reaction flask, reflux to separate water, and react at 110°C for 15 hours, then cool down to 100°C, add 48ml of purified water and 48ml of ethyl acetate, heat up, reflux for 10 minutes, separate layers after cooling down, cool the water layer to 15-20°C for crystallization for 24 hours, filter, rinse with ethyl acetate and acetone to obtain hydrochloric acid Phentolamine 8.5g, properties: brown solid, HPLC 99%, melting point 244°C-249°C, yield 66.93%.
Embodiment 2
[0033] Preparation of Phentolamine
[0034] Add 8.5 g (0.027 mol) of phentolamine hydrochloride and 170 ml of purified water into the reaction flask in Example 1, heat up to 80° C. to completely dissolve, adjust the pH to 8 with aqueous sodium carbonate solution, add 0.7 g of activated carbon, and keep warm for 20 minutes for decolorization , filtered, the filtrate was cooled to 10°C, adjusted to pH = 9.5 with NaOH aqueous solution, kept at 5°C for crystallization for 4 hours, filtered, washed with purified water until neutral, and 6.5g of phentolamine was obtained. Properties: off-white solid, HPLC 98% , melting point 165°C-168°C, yield 85.56%.
Embodiment 3
[0036] Preparation of Phentolamine Mesylate:
[0037] Add 6.5g (0.023mol) of phentolamine and 52ml of isopropanol into the reaction flask in Example 2, slowly add methanesulfonic acid dropwise at 50-60°C to adjust the pH to 6, cool down to 10°C-15°C, and keep warm Crystallize for 4 hours, filter, rinse with ethyl acetate to obtain 7.4 g of crude phentolamine mesylate, properties: off-white solid, HPLC 98%, melting point 176°C-180°C, yield 85.24%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com