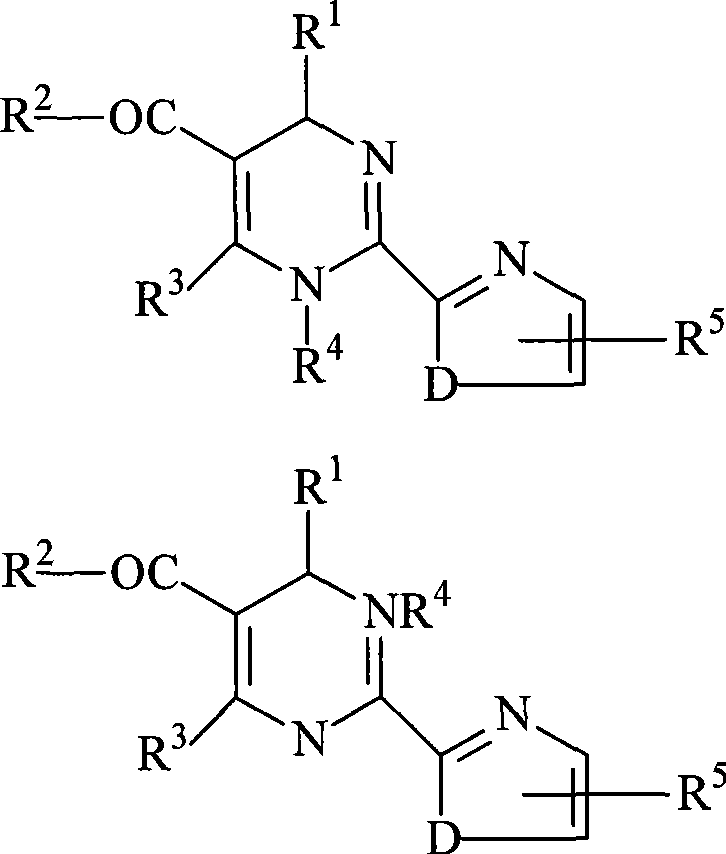
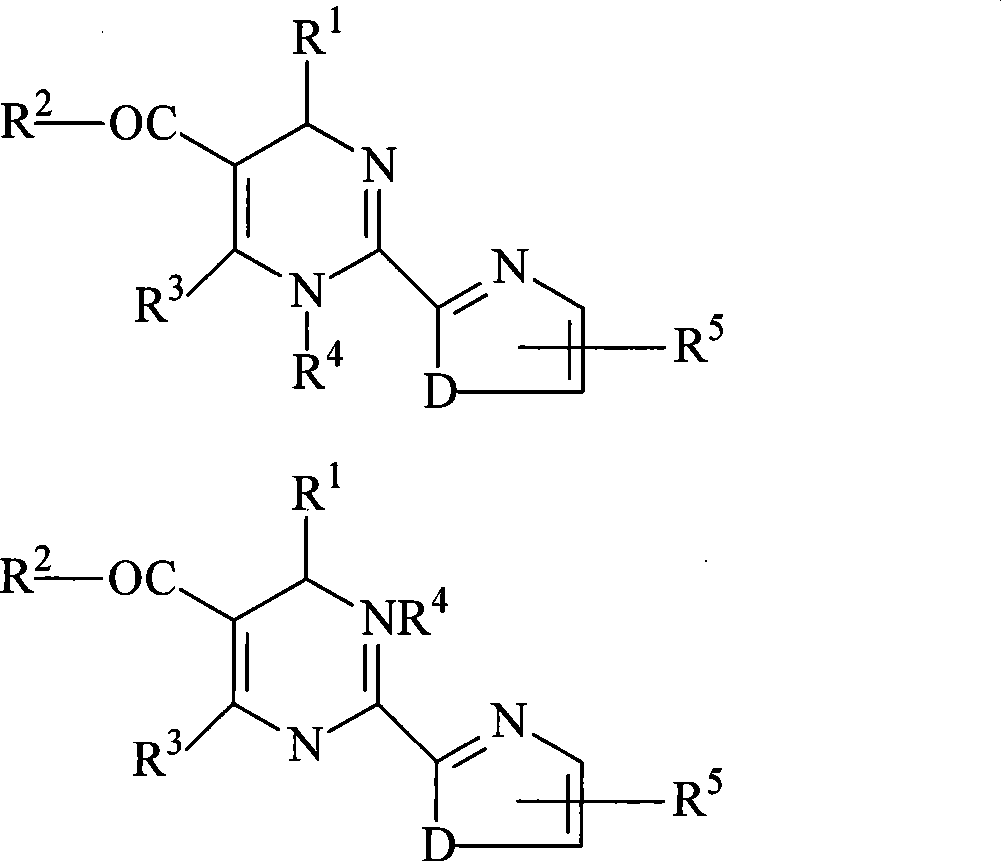
Method for splitting 2-heterocycle substituted dihydropyrimidine racemic compound
A technology of dihydropyrimidine and compound is applied in the field of splitting of racemic compounds, and achieves the effects of strong practicability, high yield and stable splitting method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
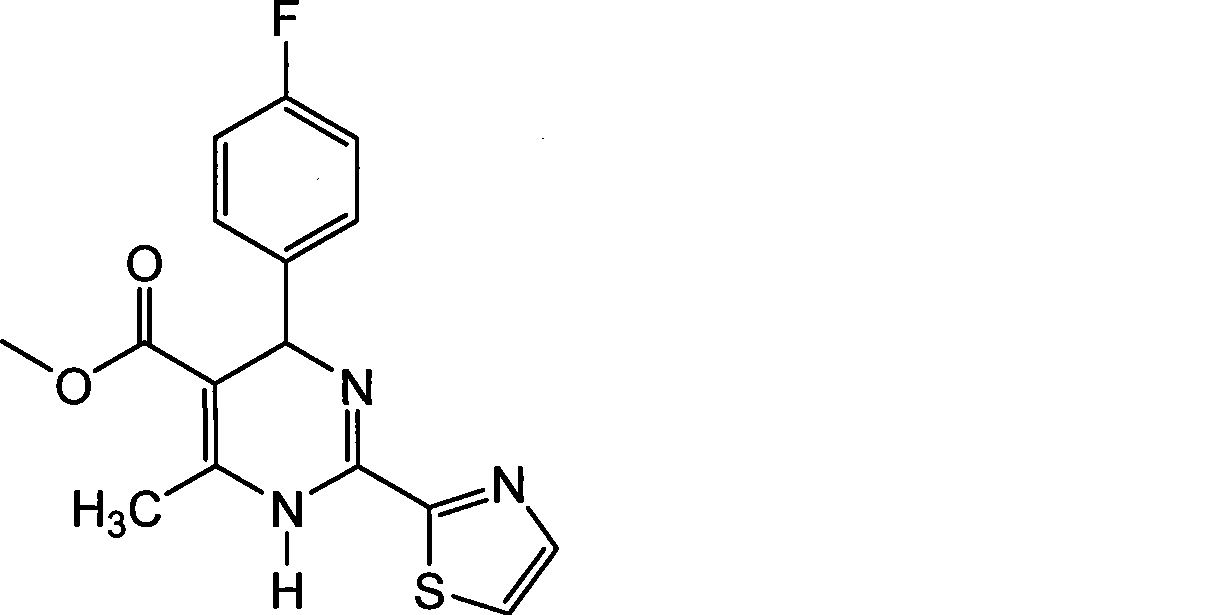
[0033] (1) 1.0 mol of the racemic compound of 4-(4-fluorophenyl)-6-methyl-2-(thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylic acid methyl ester, Its structural formula is as follows:
[0034]
[0035] and 0.50mol L-binaphthol phosphate are dropped into the reactor, and 2.5L dichloromethane and 2.5L sherwood oil are added successively under mechanical stirring;
[0036] (2) Stirring at room temperature for 0.5h, left and right rotation of methyl 4-(4-fluorophenyl)-6-methyl-2-(thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylate Fully combined with L-binaphthol phosphate resolving agent to form L-4-(4-fluorophenyl)-6-methyl-2-(thiazol-2-yl)-1,4-dihydropyrimidine-5 -Methyl carboxylate L-binaphthol phosphate and D-4-(4-fluorophenyl)-6-methyl-2-(thiazol-2-yl)-1,4-dihydropyrimidine-5- Methyl carboxylate L-binaphthol phosphate;
[0037] (3) filtering, concentrating the mother liquor to 0.2 times the original volume, and then placing the concentrated mother liquor at 0°C overnight;...
Embodiment 2
[0041] (1) 1.0mol 4-(2-bromo-4-fluorophenyl)-6-methyl-2-(thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylic acid ethyl ester The racemic compound, its structural formula is as follows:
[0042]
[0043] Put into reactor with 1.0mol L-binaphthol phosphate ester, add 4.5L dichloromethane and 32L sherwood oil successively;
[0044] (2) Stirring at room temperature for 1.0h, rotating 4-(2-bromo-4-fluorophenyl)-6-methyl-2-(thiazol-2-yl)-1,4-dihydropyrimidine-5- Ethyl carboxylate and L-binaphthol phosphate resolving agent are fully combined to form L-4-(2-bromo-4-fluorophenyl)-6-methyl-2-(thiazol-2-yl)-1 , Ethyl 4-dihydropyrimidine-5-carboxylate L-binaphthol phosphate and D-4-(2-bromo-4-fluorophenyl)-6-methyl-2-(thiazol-2-yl )-1,4-dihydropyrimidine-5-carboxylic acid ethyl ester L-binaphthol phosphate;
[0045] (3) Filtration, concentrating the mother liquor to 0.5 times the original volume, and then placing the concentrated mother liquor at -5°C for 8 hours;
[0046](4) and the...
Embodiment 3
[0049] (1) 0.40mol 4-(2-bromo-4-fluorophenyl)-6-methyl-2-(thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylic acid ethyl ester The racemic compound and 1.2mol d-binaphthol phosphate are dropped into the reactor, and 2.5L acetone and 50L hexanaphthene are added successively;
[0050] (2) Stir at room temperature for 3 hours, rotate 4-(2-bromo-4-fluorophenyl)-6-methyl-2-(thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylate Acetate ethyl ester is fully combined with D-binaphthol phosphate resolving agent to form L-4-(2-bromo-4-fluorophenyl)-6-methyl-2-(thiazol-2-yl)-1 , 4-dihydropyrimidine-5-carboxylic acid ethyl ester dex-binaphthol phosphate and dex-4-(2-bromo-4-fluorophenyl)-6-methyl-2-(thiazole-2- Base)-1,4-dihydropyrimidine-5-carboxylic acid ethyl ester D-binaphthol phosphate;
[0051] (3) Filtrate, concentrate the mother liquor to 0.6 times the original volume, and then place the concentrated mother liquor at 25°C for 48h;
[0052] (4) and then filtered, and then the filtered ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com