Preparation of lithium iron phosphate precursor and charging battery electrode thereof
A precursor and electrode technology, applied in the field of rechargeable batteries, can solve the problems of reduced active material occupancy, unsatisfactory battery performance, slowed down reaction speed, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
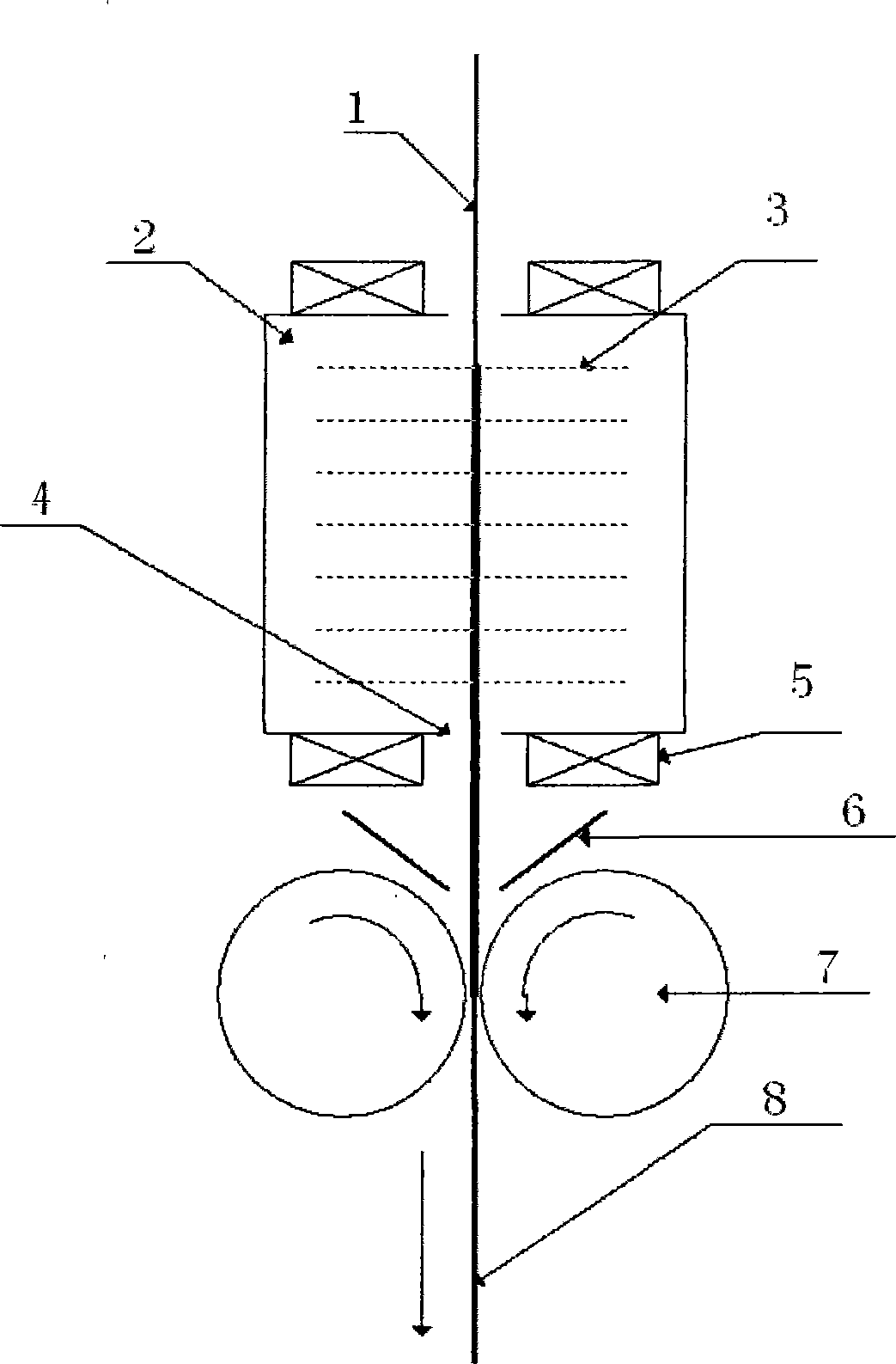
[0028] Embodiment one: see figure 1 , figure 2 , a preparation method of a rechargeable battery electrode, by first preparing a precursor, and then using the precursor to directly prepare an electrode, the specific steps are as follows:
[0029] 1. Precursor preparation:
[0030] (1) LiOH·H 2 O, Fe(NO 3 ) 3 9H 2 O, H 3 PO 4 , Ascorbic acid is weighed in a molar ratio of 1:1.2:1:0.6, LiOH·H 2 O is dissolved in deionized water to obtain a solution, the solution concentration is 8.0mol / L, add ascorbic acid, add Fe(NO 3 ) 3 9H 2 O, add H at the end 3 PO 4 solution, fully stirred and mixed evenly, aged for 2 hours;
[0031] (2) Dry for 10 hours at a temperature of 70°C and a vacuum of less than 10 Pa;
[0032] (3) The dried product was ground in a ball mill for 30 min, then placed in an alumina crucible, and heat-treated at 210° C. for 2 hours under the protection of argon to obtain a precursor.
[0033] 2. Electrode preparation method:
[0034] (1) Mix the prepare...
Embodiment 2
[0039] Embodiment two: see figure 1 , figure 2 .
[0040] 1. Precursor preparation:
[0041] (1) Li(CH 3 CO 2 )·2H 2 O, Fe(NO 3 ) 3 9H 2 O. Li 3 PO 4 、HOCH 2 COOH is weighed in a molar ratio of 1:1.2:0.8:1.2, Li(CH 3 CO 2 )·2H 2 O was dissolved in deionized water to obtain a 5.0mol / L solution, and then HOCH was added 2 COOH, add Fe(NO 3 ) 3 9H 2 O, add Li at the end 3 PO 4 solution, fully stirred and mixed evenly, and aged for 4 hours;
[0042] (2) Dry for 11 hours at a temperature of 80°C and a vacuum of 9 Pa;
[0043] (3) Add 6% sucrose of the product weight to the dried product, grind it in a ball mill for 40 minutes, then place it in a graphite crucible, and heat-treat it at 300° C. for 2 hours under the protection of nitrogen to obtain a precursor.
[0044] 2. Electrode preparation:
[0045] (1) Mix the precursor and the graphite conductive agent evenly in a weight ratio of 1:0.08, and put them into the powder box 2;
[0046] (2) Put the perforated a...
Embodiment 3
[0050] Embodiment three: see figure 1 , figure 2 .
[0051] 1. Precursor preparation:
[0052] (1) Li(CH 3 CO 2 )·2H 2 O, Fe(NO 3 ) 3 9H 2 O. Li 3 PO 4 、C 2 h 2 o 4 Weigh it according to the molar ratio of 1:1.2:1.1:0.5, Li(CH 3 CO 2 )·2H 2 O was dissolved in deionized water to obtain a solution with a concentration of 4.0mol / L, and then C was added 2 h 2 o 4 , add Fe(NO 3 ) 3 9H 2 O, add Li at the end 3 PO 4 solution, fully stirred and mixed evenly, aged for 2 hours;
[0053] (2) Dry for 6 hours at a temperature of 90°C and a vacuum of 5 Pa;
[0054] (3) Add 8% glucose by weight to the dried product, grind it in a ball mill for 30 min, then place it in a graphite crucible, and heat-treat it at 300° C. for 2 hours under the protection of nitrogen to obtain a precursor.
[0055] 2. Electrode preparation:
[0056] (1) Mix the precursor and the carbon black conductive agent evenly in a weight ratio of 1:0.1, and put them into the powder box 2;
[0057]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com