Diethoxy thiophosphate organophosphorus pesticide hapten and preparation thereof
A technology of diethoxy phosphorothioate and diethoxy phosphorothioate, applied in the field of immunology, can solve problems such as not being well solved, and achieve the effect of good specificity and high titer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
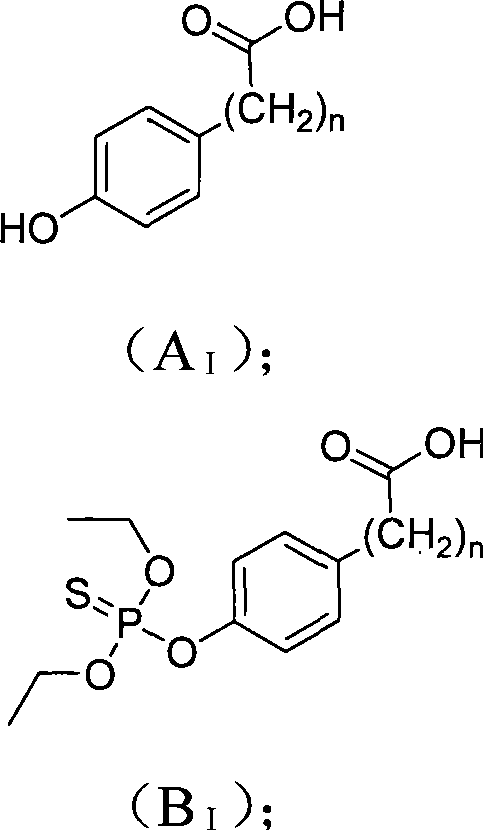
[0029] Example 1 Hapten B I (n=0) preparation method
[0030] At 0°C, 0.884g (6.4mmol) of p-hydroxybenzoic acid and 0.898g (16mmol) of KOH were dissolved in 50mL of methanol. While stirring, 1.81 g (9.6 mmol) of diethoxyphosphoryl thiochloride was added dropwise, the temperature was raised to 65°C, and the reaction was stirred for 18 hours. After the reaction was completed, the reaction was concentrated by rotary evaporation, and the obtained crude product was dissolved in ethyl acetate, extracted with 50 mL of 2M NaOH solution, the aqueous layer was acidified with 6M hydrochloric acid, and precipitated, washed with water to obtain 1.05 g of the product, with a yield of 56.4% . ESI-MS analysis (negative) m / z 289[M-H]-; 1H-NMR (400MHz, CDCl 3 and TMS): δ 1.36 (t, J=7.1Hz, 6H, CH 3 ); 4.24(qd,J 1 =7.1Hz,J 2 =7.1Hz,J 3 =7.1Hz,J 4 =10.0Hz, 4H, CH 2 ); 7.26 (d, J=8.1, 2H, ArH); 8.09 (d, J=8.5, 2H, ArH).
Embodiment 2
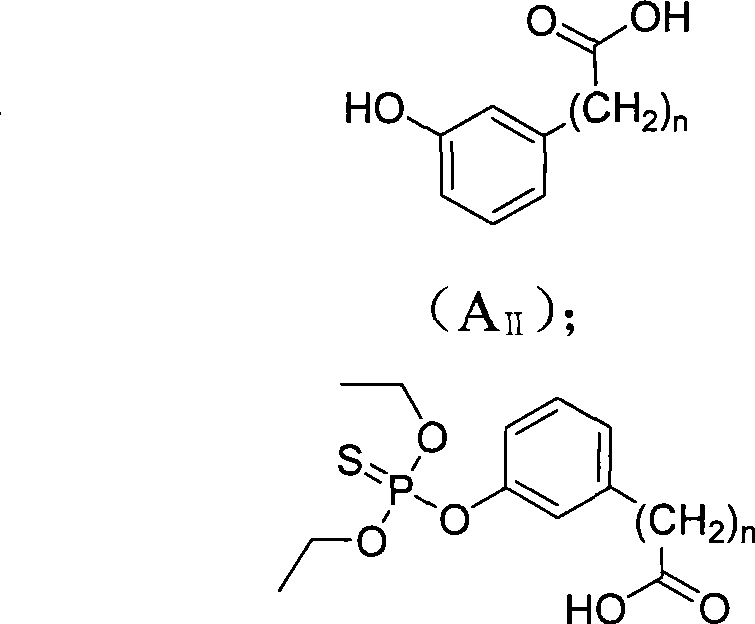
[0031] Example 2 Hapten B II (n=0) Preparation method
[0032]At 0°C, 0.884g (6.4mmol) of m-hydroxybenzoic acid and 0.898g (16mmol) of KOH were dissolved in 50mL of methanol. While stirring, 1.81 g (9.6 mmol) of diethoxyphosphoryl thiochloride was added dropwise, the temperature was raised to 65°C, and the reaction was stirred for 18 hours. After the reaction was completed, the reaction was concentrated by rotary evaporation, and the obtained crude product was dissolved in ethyl acetate, the solution was concentrated, and chloroform:methanol=8:1 was passed through a silica gel column to obtain 0.5 g of a yellow solid with a yield of 27.0%. ESI-MS analysis (negative) m / z 289[M-H]-; 1H-NMR (400MHz, CDCl 3 and TMS): δ1.37 (t, J=7.0Hz, 6H, CH 3 ); 4.25(qd, J 1 =7.1Hz,J 2 =7.1Hz,J 3 =7.1Hz,J 4 =9.6Hz, 4H, CH 2 ); 7.45 (d, J=4.7, 2H, ArH); 7.89 (s, 1H, ArH); 7.94 (d, J=4.6, 1H, ArH).
Embodiment 3
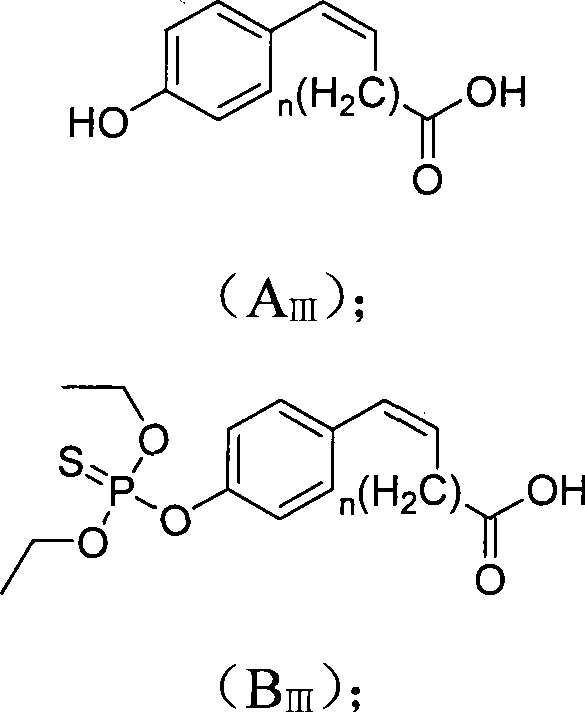
[0033] Example 3 Hapten B III (n=0) Preparation method
[0034] Take 4.8848g (0.04moL) of p-hydroxybenzaldehyde and dissolve it in pyridine, add a catalyst amount of DMAP, protect it with a drying tube, put it in a water bath at 85°C, and stir. Add 3.746 g (0.036 moL) of malonic acid dropwise to the above solution and react for 3 h. After the reaction was completed, the mixture was poured into a saturated sodium bicarbonate solution, stirred for 15 min, extracted with ethyl acetate, and the pH of the aqueous layer was adjusted to 3 with 4M hydrochloric acid, and 2.32 g of a white solid was precipitated, with a yield of 78.6%. ESI-MS analysis (negative) m / z 163[M-H]-; 1H-NMR (400MHz, d6-Acetone and TMS): δ 6.33 (d, J=16.0Hz, 1H, CH=CH); 6.89 (d, J=8.6Hz, 2H, ArH); 7.54(d, J=8.6Hz, 2H, ArH); 7.60(d, J=16.0Hz, 1H, CH=CH); 8.86(s, 1H, OH); 10.77 (s, 1H, COOH).
[0035] Weigh 0.7 (0.0125 moL) KOH and dissolve it in anhydrous methanol, weigh 0.821 g (0.005 moL) of the above soli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com