Trifluralin haptens, as well as preparation method and application thereof
The technology of a hapten and trifluralin, which is applied in the field of immunology, can solve the problems of lack of trifluralin, etc., and achieve the effect of simple and feasible preparation method, high purity and yield, and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
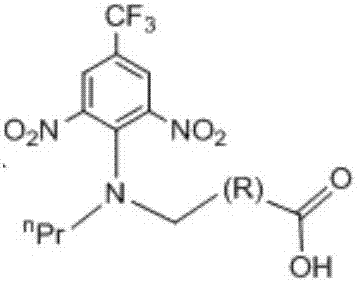
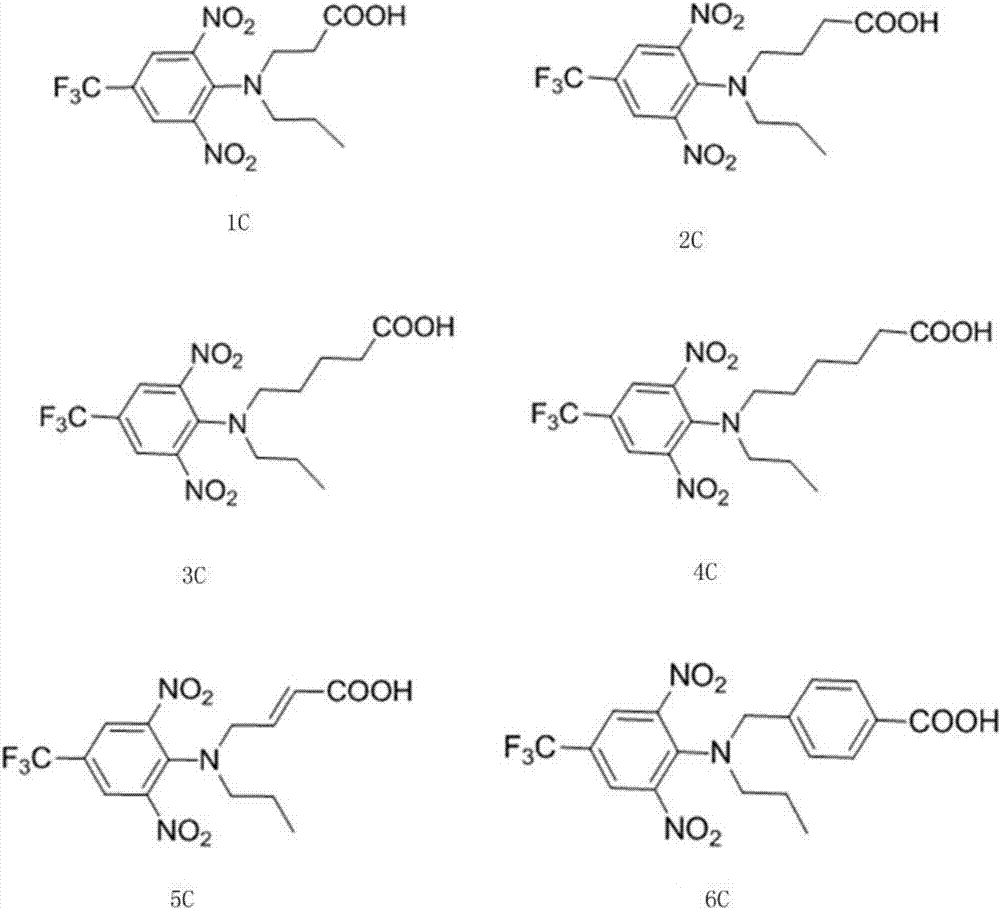
[0042] Embodiment 1: The synthesis method of trifluralin hapten compound 1c is as follows:
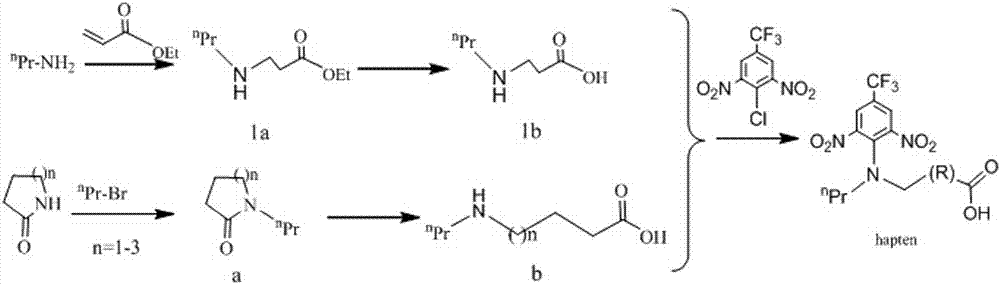
[0043] (1) Ethyl acrylate (3600mg, 36mmol) was dissolved in 30mmol of chloroform, and n-propylamine (3191.94mg, 54mmol) was added in an oil bath and stirred at 40°C for 12h to obtain the ester group-containing secondary amine intermediate 1a.
[0044] (2) Dissolve 1a (239mg, 1.5mmol) in 6ml THF, add NaOH aqueous solution (120mg, 3mmol), stir at room temperature, and perform hydrolysis to obtain 1b after hydrolysis.
[0045] (3) Add 3,5-dinitro-4-chloro-trifluorotoluene (270 mg, 1 mmol) directly to 1b, and stir at room temperature. Use a chromatographic solution with a ratio of n-hexane: ethyl acetate = 2:1 to track the plate. After the reaction is completed, perform acid-base extraction, combine the acid-extracted organic phases, and dry with anhydrous sodium sulfate. Spin-dried at 40°C, and separated on a silica gel plate with a chromatographic solution of n-hexane: ethyl acetate: ac...
Embodiment 2
[0046] Embodiment 2: The synthesis method of trifluralin hapten compound 2c is as follows:
[0047] (1) 2-Pyrrolidone (1000mg, 11mmol) was dissolved in 10ml of DMF, 13.2mmol of NaH (60%) and n-bromopropane (1758.9mg, 14.3mmol) were added, and stirred overnight in an oil bath at 70°C under nitrogen protection. Add 70ml of ethyl acetate to the reaction solution, extract with 50ml of saturated ammonium chloride solution, combine the organic phases, and dry over anhydrous sodium sulfate. Spin-dry at 40°C to obtain the ester group-containing secondary amine intermediate 2a.
[0048] (2) 2a (846mg, 6mmol) was added to 13ml 4N NaOH aqueous solution and stirred in an oil bath at 100°C overnight for hydrolysis to obtain 2b.
[0049] (3) Dissolve 3,5-dinitro-4-chloro-trifluorotoluene (270 mg, 1 mmol) in 6 ml of tetrahydrofuran, add 2b (190.5 mg, 1.5 mmol), and stir at room temperature. Dilute with 30ml of water, add 1M hydrochloric acid to adjust the pH to 2-3, and a precipitate appea...
Embodiment 3
[0050] Embodiment 3: The synthesis method of trifluralin hapten compound 3c is as follows:
[0051] (1) Dissolve valerolactam (2180mg, 20mmol) in 10ml DMF, add 24mmol NaH (60%), n-bromopropane (2950mg, 24mmol), stir in an oil bath at 70°C, and react under nitrogen protection for 4-5 hours. Dilute with 50ml of water, add 1M hydrochloric acid to adjust the pH to 2-3, and a precipitate appears. Extracted twice with ethyl acetate, the amount of ethyl acetate used each time was 50ml, the precipitate was dissolved, washed with 15ml of saturated brine, the organic phases were combined, and dried over anhydrous sodium sulfate. After spin-drying at 40°C, the secondary amine 3a containing an ester group was obtained, and the yield was 55% by weight.
[0052] (2) Add 2.5ml of 37% hydrochloric acid and 2.5ml of water to 3a (846mg, 6mmol), condense and reflux in an oil bath at 100°C for 4-5 hours for hydrolysis, and obtain the hydrolyzate 3b.
[0053] (3) Dissolve 3,5-dinitro-4-chloro-tr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com