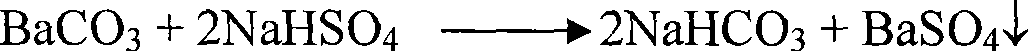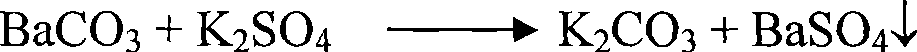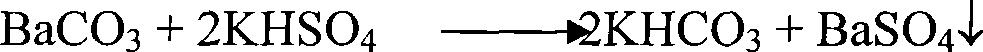Method for producing barium sulfate
A production method and barium sulfate technology, applied in the direction of calcium/strontium/barium sulfate, etc., can solve problems affecting product quality, etc., and achieve the effects of high comprehensive utilization of resources, good economic benefits, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Weigh 100g of industrial barium carbonate, add 120mL of water to slurry with strong stirring for 2 hours, add 100g of anhydrous sodium sulfate at 60°C and stir for 2 hours, filter, and add the filter cake to dilute sulfuric acid with a pH value of 0.5 at a solid-to-liquid ratio of 1:2 In the solution, stir and wash at 80°C for 0.5h, then filter, wash, and dry to obtain precipitated barium sulfate with a purity of 98.3%. Washing water and barium carbonate transformation solution are mixed and stirred, and sulfuric acid is added to adjust the pH value to 6.6 to convert it into a mixed solution of sodium sulfate and sodium bisulfate, which is used as a barium carbonate slurry transformation solution, and the released CO 2 It is used as raw material for the production of barium carbonate.
Embodiment 2
[0044] The mixed solution of sodium sulfate and sodium bisulfate obtained in Example 1 was added to 100 g of barium carbonate powder ground to minus 120 mesh, stirred at 30°C for 6 hours, filtered, and the filter cake was added with sulfuric acid at a solid-to-liquid ratio of 1:3 to a concentration of 10 wt. % aqueous solution at 50°C for 1 h, then filtered, washed, and dried to obtain precipitated barium sulfate with a purity of 98.5%. Add appropriate amount of water to the washing water so that the concentration of sulfuric acid is 8% for standby; after transformation, the liquid is fed into CO 2 Adjust the pH value to 8.0, and keep it at 15°C for 1.5 hours to crystallize and precipitate NaHCO 3 , filter, and pyrolyze NaHCO at 240°C 3 Sodium carbonate is obtained; the crystallization mother liquor is used as the next batch of barium carbonate slurry.
Embodiment 3
[0046] Weigh 100g of industrial barium carbonate, stir it into 120mL of water for 2 hours, add 120g of anhydrous potassium sulfate at 50°C, stir for 4 hours, filter, add the filter cake to the washing water prepared in Example 2 according to the solid-to-liquid ratio of 1:4 Stir and wash at 35°C for 2.5 hours, then filter, wash, and dry to obtain precipitated barium sulfate with a purity of 98.4%. Washing water and barium carbonate transformed solution are mixed and stirred, and sulfuric acid is added to adjust the pH value to 6.8 to convert it into a mixed solution of potassium sulfate, potassium bisulfate, sodium sulfate, and sodium bisulfate, which is used as the slurry transformation liquid of barium carbonate, and the emitted CO 2 It is used as raw material for the production of barium carbonate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com