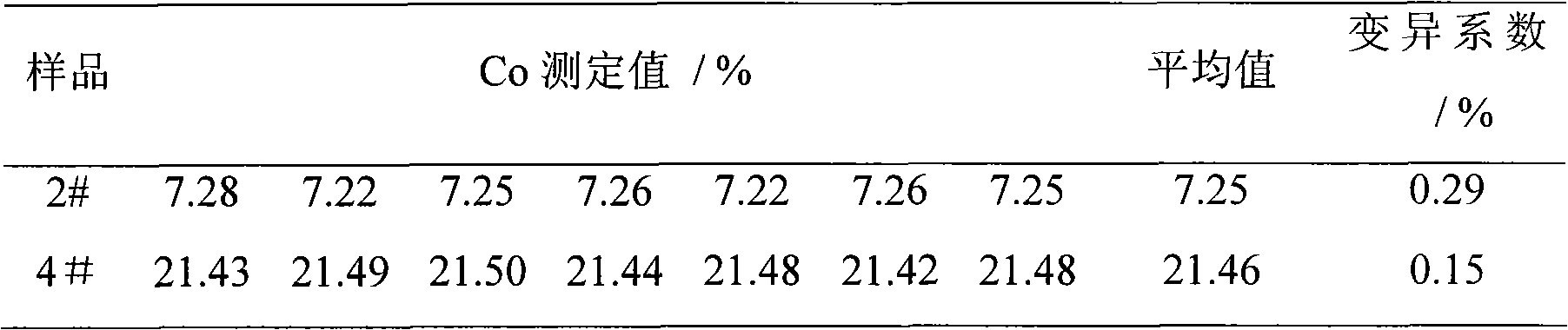
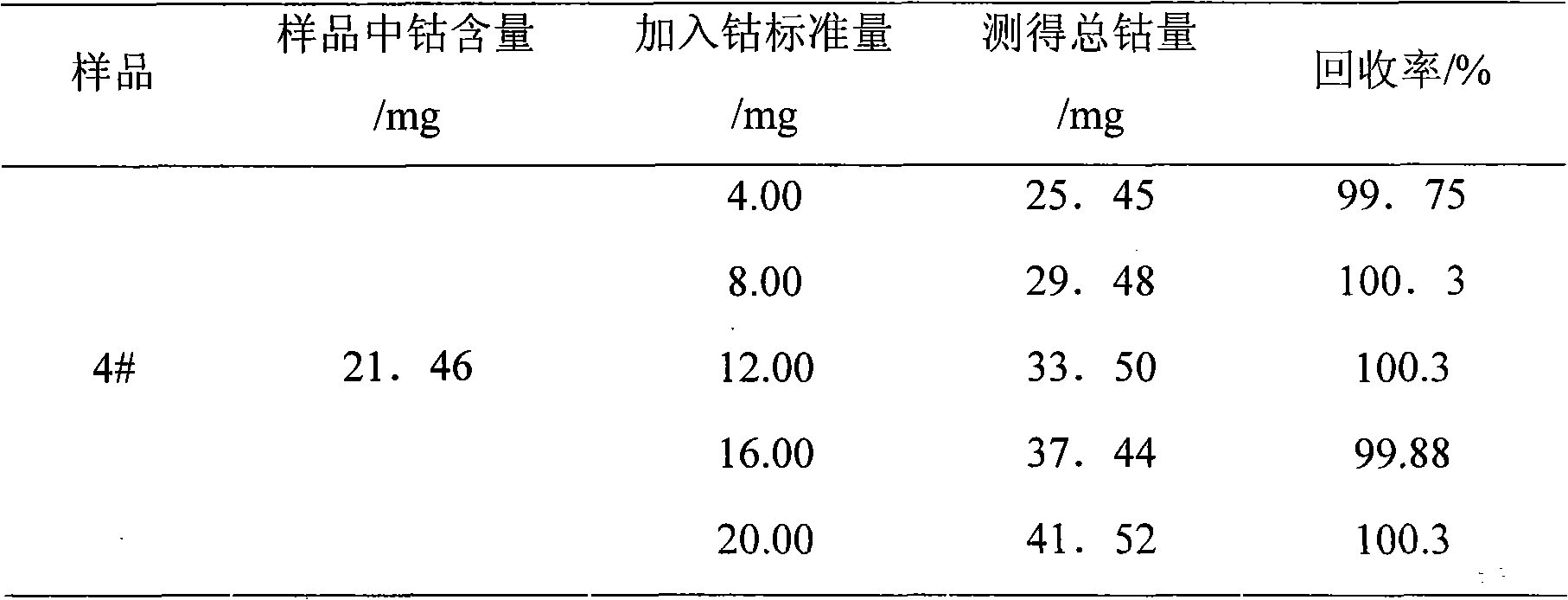
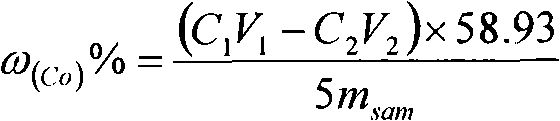Chemical assay method for cobalt in lithium ion battery anode material LiCoxMnyNi1-x-yO2
A technology for chemical determination and manganese dioxide, applied in chemical analysis by titration, chemical analysis by precipitation, etc., can solve the problem of reducing cation mixing between Li layer and transition metal layer, reducing reversible lithium intercalation capacity, and operating requirements. High problems, to achieve the effect of shortened measurement cycle, sharp color change, and high measurement accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] The present invention will be further described in detail below in conjunction with the examples.
[0024] Embodiment of the invention
[0025] 1. Standard solution preparation and calibration
[0026] (1)C (1 / 6KIO3) =0.1000mol / L potassium iodate standard solution:
[0027] Preparation: Accurately weigh 0.8917g of the standard potassium iodate with an analytical balance, prepare 250mL aqueous solution with a volumetric flask, and shake well for later use.
[0028] (2) Sodium arsenite solution:
[0029] Preparation: weigh NaAsO 2 1.0 g of the solid was dissolved in 500 mL of water.
[0030] Calibration: draw 25mLKIO with a pipette 3 3 parts of standard solution were placed in 250mL Erlenmeyer flasks respectively, and 20mL of 100g / L KI solution, 5mL of 1mol / L H 2 SO 4 , the solution is brownish red at this time, add an appropriate amount of ammonia water to adjust the pH of the solution to weak alkaline, NaAsO 2 Titration, when the solution is pale yellow, add 1m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com