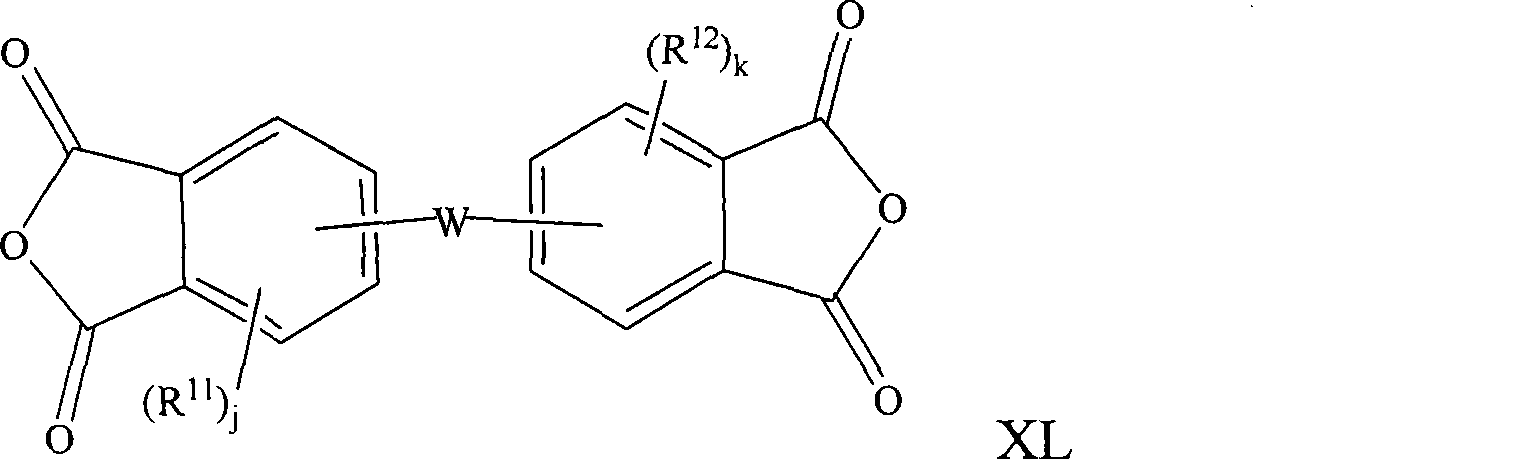
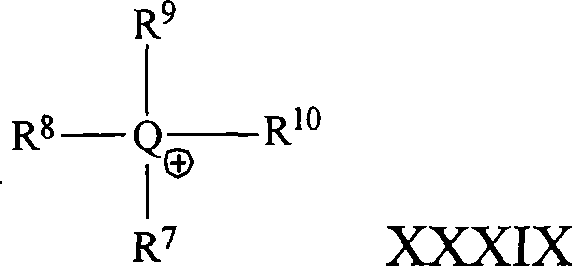Compositions and methods for polymer composites
A composite composition, polymer technology, applied in the open field, can solve problems such as adverse interactions, marginal performance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0371] Example 1 Preparation of (3-aminophenyl) triphenylphosphonium iodide 1
[0372]
[0373] Into a 3000 mL three-neck round bottom flask equipped with a condenser, a mechanical stirrer and a gas inlet, add about 329.33 g (1.25 mol) of triphenylphosphine (PPh 3 ), Pd(CH 3 COO) 2 (2.82 g, 0.0126 mol) and 1600 mL of degassed xylene. The mixture was stirred under argon until PPh3 dissolved. m-Iodoaniline (about 275.00 g, 1.25 mol) was added and the yellow-orange solution was refluxed for about 80 minutes. The product phosphonium compound ((3-aminophenyl)triphenylphosphonium iodide) was isolated from solution as a yellow-orange solid. Avoid excessive reflux to prevent discoloration of the product phosphonium compound. The progress of the reaction was monitored by thin layer chromatography (TLC) using a 50 / 50 hexane / ethyl acetate developing solution. After reflux, the product was filtered. Product 1 was reslurried with hot toluene and stirred for 15 minutes. The solut...
Embodiment 2
[0374] Example 2 Preparation of 4-(4-cumyl)-phenoxy-phthalonitrile 2
[0375]
[0376] Add 4-cumylphenol (170.9 g, 0.80 moles), 4-nitrophthalonitrile (150 g, 0.87 moles), potassium carbonate (155.8 g, 1.13 moles), and dimethylformaldehyde to a 3-liter flask Amide (1.4 L). The solution was heated to about 90°C under nitrogen with stirring for about 100 minutes. The progress of the reaction was monitored by thin layer chromatography. The dark brown reaction mixture was cooled and 2M HCl solution (600 mL) was added with stirring. The organic layer was extracted with chloroform (3 x 300 mL). The chloroform layer was separated, washed with water (3×100 mL), and dried (MgSO 4 ). The mixture was filtered and the solvent was evaporated on a hot oil bath at a temperature greater than about 100 °C to afford the crude nitrile 2 as a viscous green oil (278 g, 84% yield). 1 H NMR (δ, D 6 -DMSO): 8.09 (d, 1H), 7.78 (d, 1H), 7.40-7.15 (m, 8H), 7.10 (d, 2H), 1.66 (s, 6H, Me).
Embodiment 3
[0377] Example 3 Preparation of 4-(4-cumyl)phenoxy-phthalic anhydride 3
[0378]
[0379] A 3 L three-neck round bottom flask was equipped with a condenser, a mechanical stirrer, and an addition funnel. To the flask was added 4-(4-cumylphenoxy)-phthalonitrile (278 g, 0.82 moles) and acetic acid (1.6 L). Add 70% sulfuric acid (670 mL) to the addition funnel. The solution was heated to 120°C, and sulfuric acid was added dropwise to the reaction mixture over 2 hours. The resulting mixture was refluxed overnight (12 hours). The reaction mixture was cooled to room temperature and poured into an ice-water mixture (-1 kg). The product was extracted with ethyl acetate (3 x 300 mL). The ethyl acetate layer was separated and washed with anhydrous MgSO 4 dry. Filter the solution to remove MgSO 4 , and the solvent was removed on a rotary evaporator. The resulting brown liquid was dried overnight in a vacuum oven at 160°C. This gave the desired anhydride as a viscous brown oil ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| aspect ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com