Composite tablet capable of treating peptic ulcer and preparation method thereof
A technology for peptic ulcer and compound tablet, which is applied in the field of medicine, can solve the problems of various types, reduce the patient's oral compliance, and high oral ineffective ingredients, and achieve the effects of improving stability, reducing gastric irritation and good stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
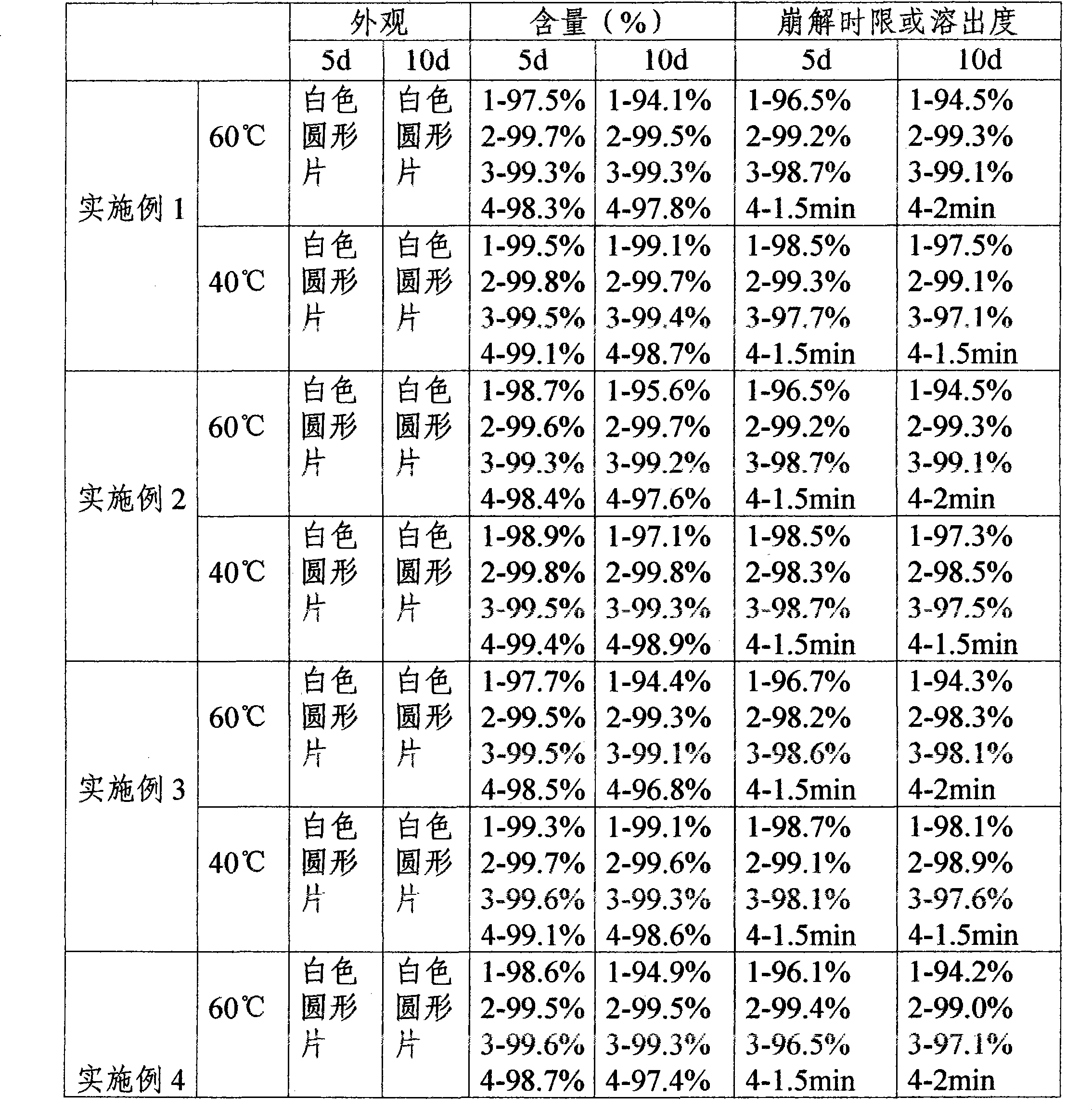
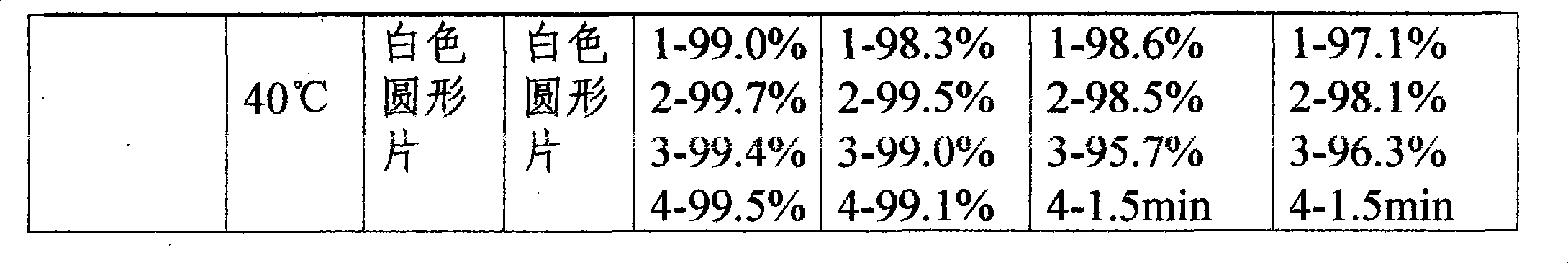
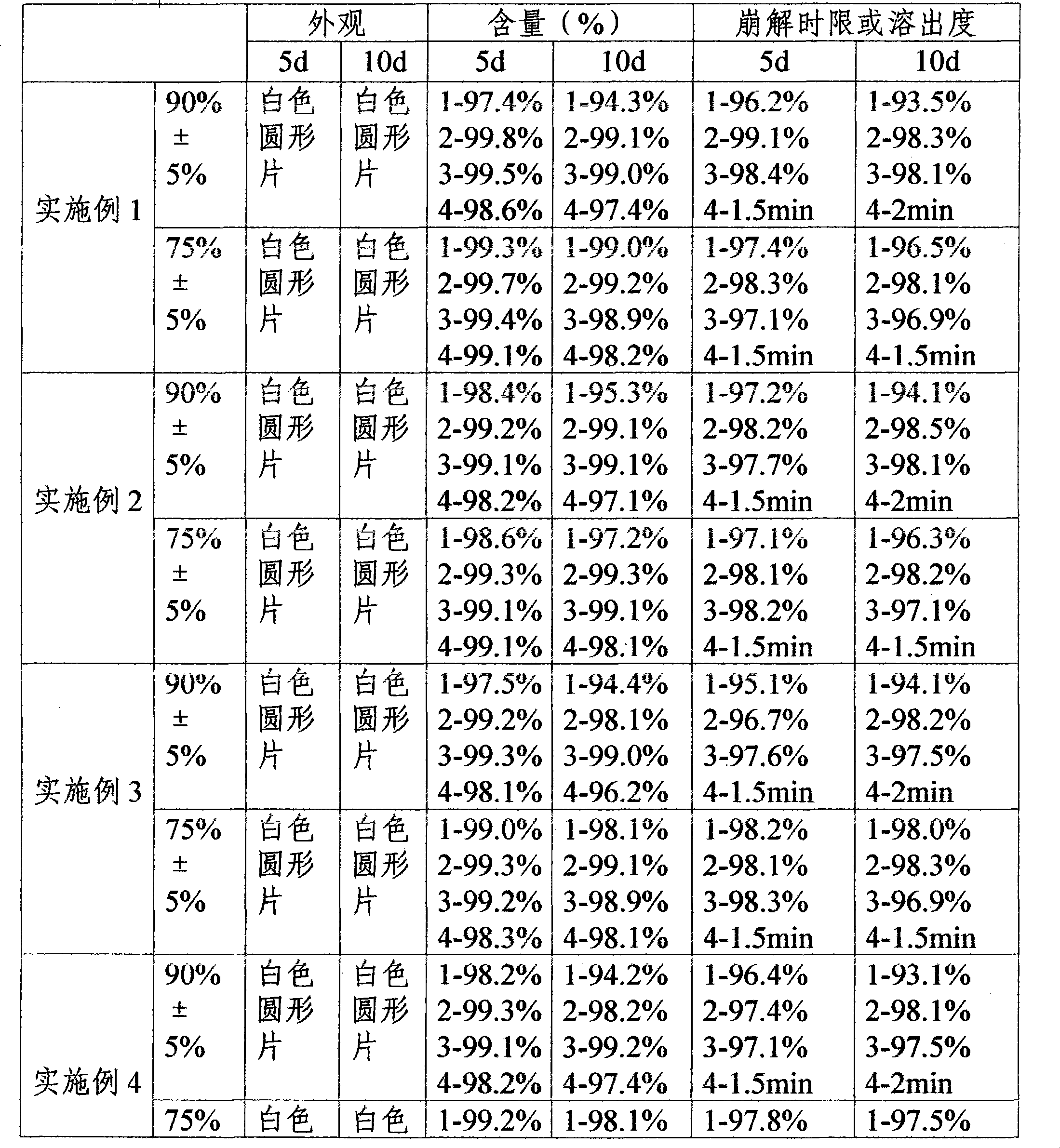
Embodiment 1
[0063] One-time prescription of compound preparation
[0064] Lansoprazole 30mg
[0065] Metronidazole 300mg
[0066] Clarithromycin 500mg
[0067] Potassium Bismuth Citrate 200mg
[0068] (1) Preparation of lansoprazole cyclodextrin inclusion compound
[0069] Lansoprazole 1 part mole fraction
[0070] HP-β-CD mole fraction 1 part
[0071] NaHCO 3 Right amount
[0072] NaOH amount
[0073] Step: Configure NaHCO 3 Adjust the pH of the 0.3mol / L solution to 12 with NaOH solution, dissolve HP-β-CD in the above solution, heat and stir at 40°C, dissolve lansoprazole with an appropriate amount of 95% ethanol and add it dropwise to the heated and stirred solution. The inclusion reaction is 2 hours. After the reaction, it was freeze-dried to obtain clathrate powder.
[0074] (2) Preparation of inclusion compound antibiotic compound tablet core
[0075] The inclusion compound is equivalent to 30g of the main drug
[0076] Metronidazole 300g
[0077] Clarithromycin 500g
[0078...
Embodiment 2
[0096] One-time prescription of compound preparation
[0097] Lansoprazole 30mg
[0098] Metronidazole 300mg
[0099] Clarithromycin 500mg
[0100] Potassium Bismuth Citrate 200mg
[0101] (1) Lansoprazole self-emulsification
[0102] Lansoprazole 30g
[0103] Ethyl oleate 5g
[0104] OP 80g
[0105] PEG400 15g
[0106] Stir and mix ethyl oleate, OP and PEG400 evenly. Dissolve lansoprazole in the mixture and set aside.
[0107] (2) Preparation of inclusion compound antibiotic compound tablet core
[0108] Lansoprazole self-emulsifying, equivalent to 30g of main drug
[0109] Metronidazole 300g
[0110] Clarithromycin 500g
[0111] MCC 700g
[0112] PVP-XL 50g
[0113] Steps: The metronidazole, clarithromycin, and MCC that have passed through a 100-mesh sieve are mixed and passed through a 100-mesh sieve, and lansoprazole is self-emulsified as a binder to prepare a soft material. Pass 80 mesh sieve to make wet granules. Dry at a low temperature of 40°C, pelletize, adjust the pressu...
Embodiment 3
[0129] One-time prescription of compound preparation
[0130] Lansoprazole 30mg
[0131] Metronidazole 300mg
[0132] Clarithromycin 500mg
[0133] Potassium Bismuth Citrate 200mg
[0134] (1) Preparation of lansoprazole cyclodextrin inclusion compound
[0135] Lansoprazole 1 part mole fraction
[0136] HP-β-CD mole fraction 1 part
[0137] NaHCO 3 Right amount
[0138] NaOH amount
[0139] Configure NaHCO 3 Adjust the pH of the 0.3mol / L solution to 12 with NaOH solution, dissolve HP-β-CD in the above solution, heat and stir at 40°C, dissolve lansoprazole with an appropriate amount of 95% ethanol and add it dropwise to the heated and stirred solution. The inclusion reaction is 2 hours. After the reaction, it was freeze-dried to obtain clathrate powder.
[0140] (2) Preparation of inclusion compound antibiotic compound tablet core
[0141] Inclusion compound equivalent to 30g of main drug
[0142] Metronidazole 300g
[0143] Clarithromycin 500g
[0144] Optimized MCC 400g-1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com