Stimulation of pancreatic Beta cell proliferation
A technology of β cells and cells, which is applied in the field of stimulating the proliferation of pancreatic β cells, and can solve the problem of increasing the mass of total islets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0178] Tcf1 regulates the expression of a novel protein in pancreatic β cells
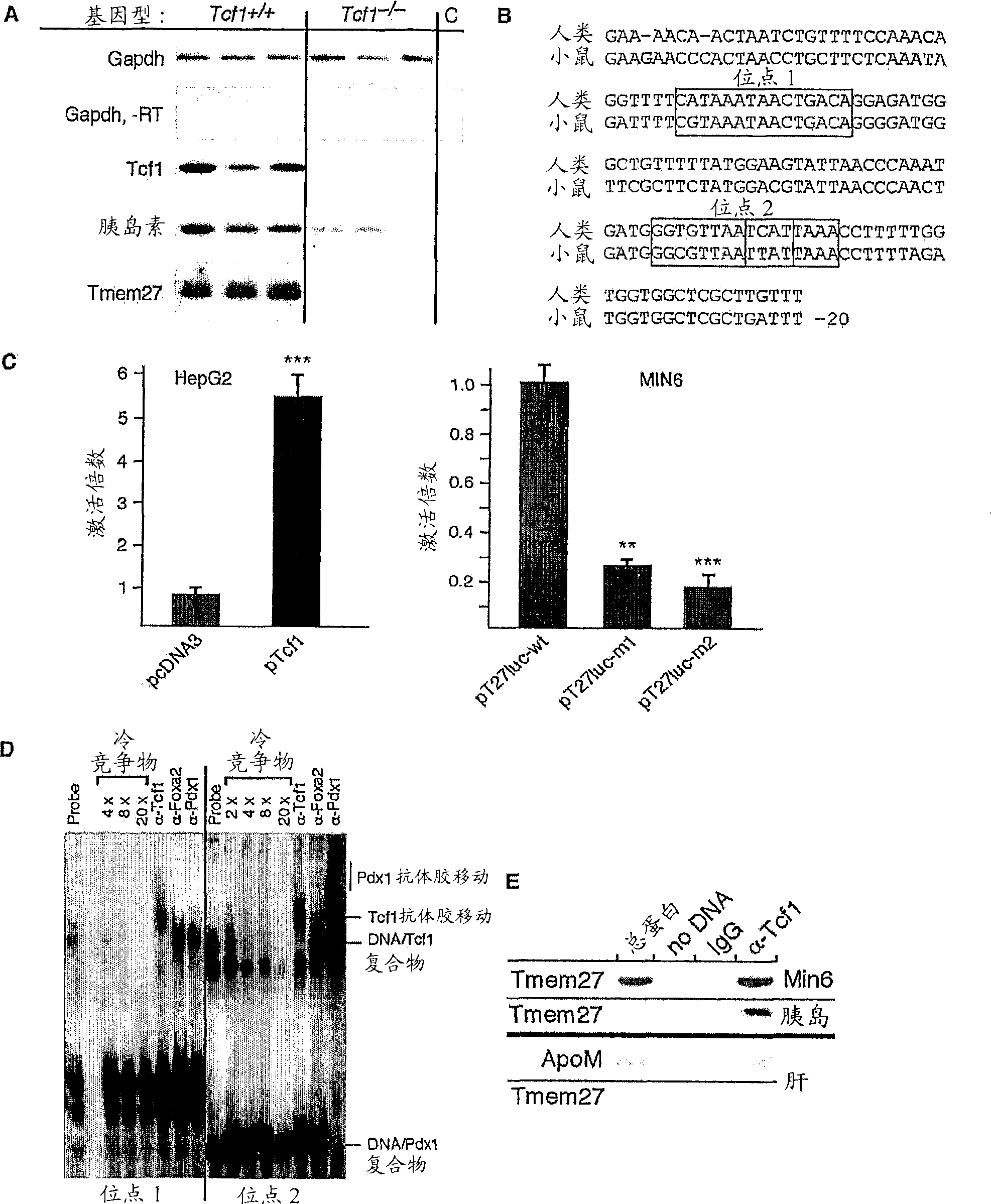
[0179] To identify mitogenic factors in pancreatic beta cells, Affymetrix TM Oligonucleotide expression assays comparing isolated Tcf1 - / - Gene expression in islets of wild-type sibling mice. The assay identified a gene encoding a transmembrane transmembrane protein (TMEM27) that expresses Tcf1 - / - Expression levels in mice were reduced 16-fold compared to controls. This result was confirmed by RT-PCR in an independent group of animals ( figure 1 a). To investigate whether Tcf1 is a direct activator of TMEM27 transcription, the TMEM27 promoter was analyzed and two conserved high-affinity binding sites were identified in its regulatory region ( figure 1 b). The human upstream regulatory region (SEQ ID NO: 10) and the mouse upstream regulatory region (SEQ ID NO: 11) comprise the Tcf binding site, starting at about -117 to about -102 in humans and mice, respectively (SEQ ID NO: 12 and SEQ ID NO...
Embodiment 2
[0182] TMEM27 expression is regulated during pancreatic development
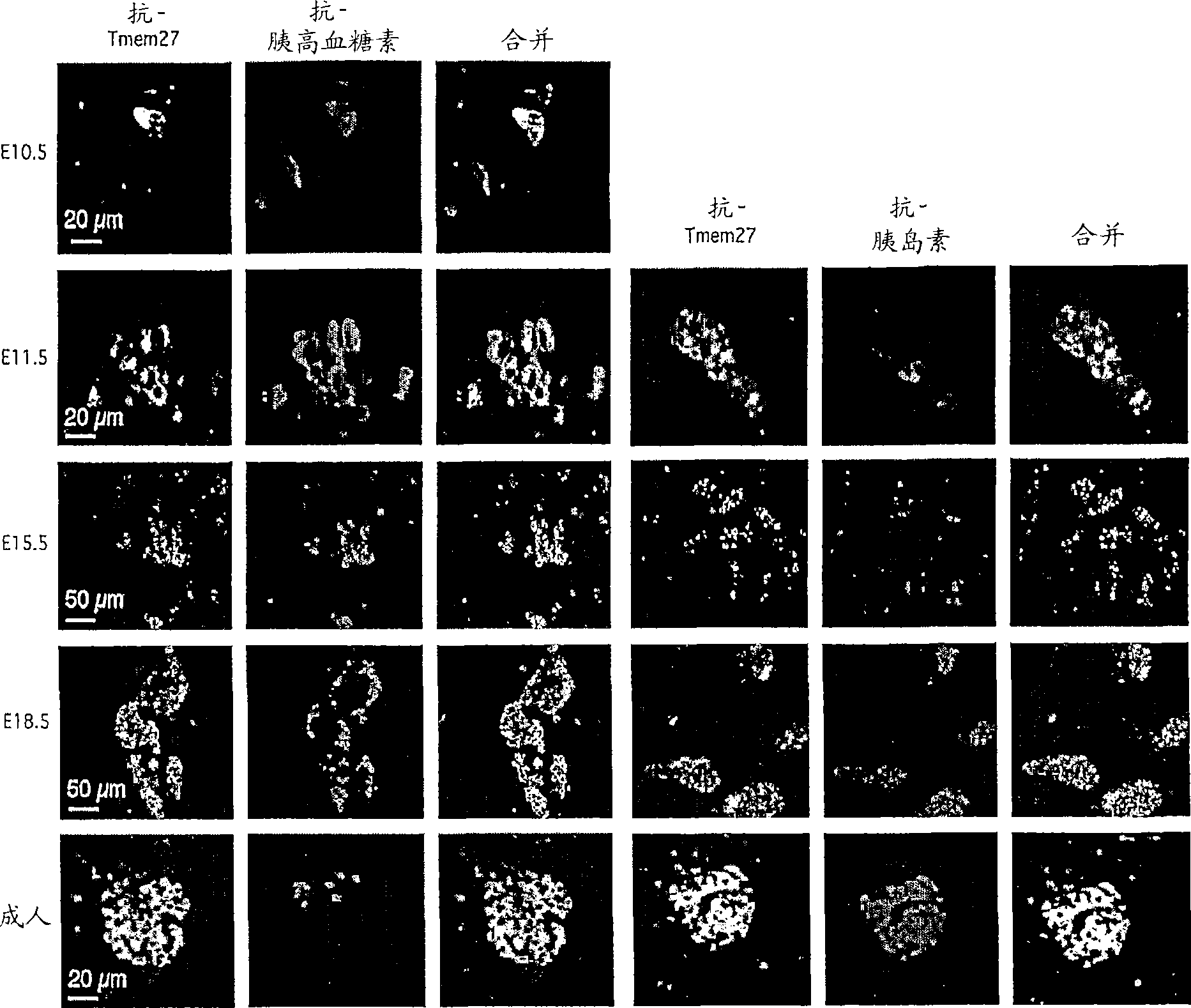
[0183] Immunohistochemical analysis showing the pattern of TMEM27 expression during mouse pancreas development. In neonatal and adult pancreas, TMEM27 expression is restricted to beta cells of islets. During pancreatic development, TMEM27 is expressed at the earliest stages when hormones (mainly glucagon-positive cells) are positive. At embryonic stages E11.5 and E12.5, TMEM27 expression is localized in glucagon- and insulin-expressing cells. At the peak of endocrine cell expansion (from embryonic stage 13.5-18.5), TMEM27 coexists predominantly with glucagon until prenatal (E18.5), when it is also detectable in insulin-positive cells. In both neonatal and adult pancreas, TMEM27 was no longer detectable in islet α-cells and its expression was restricted to β-cells ( figure 2 ).
Embodiment 3
[0185] TMEM27 is an N-linked glycoprotein that forms dimers in vivo
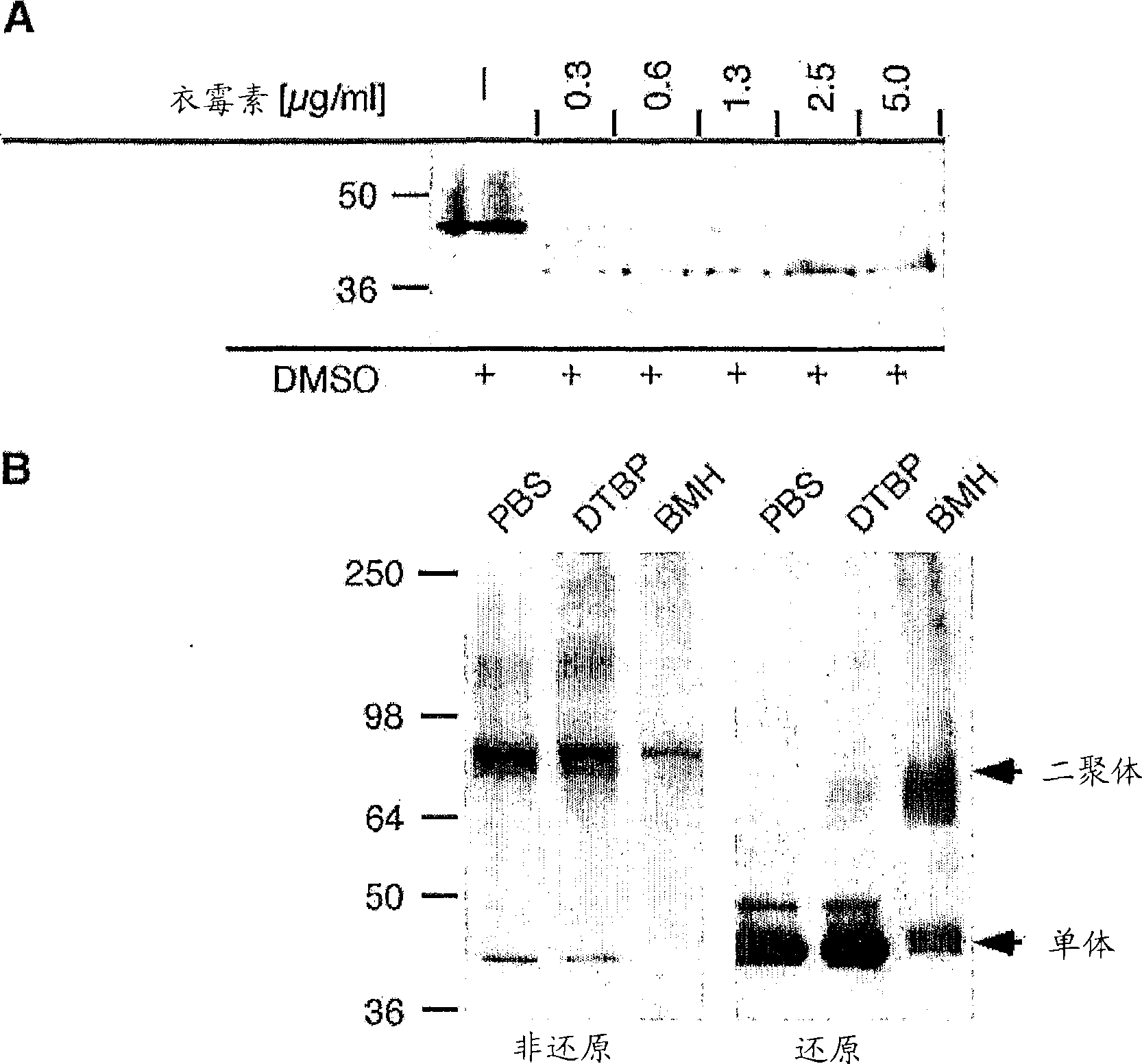
[0186] TMEM27 contains two predicted N-glycosylation sites at amino acid residues 76 and 93, respectively. MIN6 cells were treated with tunicamycin, which inhibits N-glycosylation. Cells were incubated with increasing concentrations of tunicamycin, and protein extracts were prepared and analyzed by SDS-PAGE and Western blotting. After treatment with tunicamycin, the electrophoretic mobility of the two bands increased, while the high molecular weight protein disappeared ( image 3 a). This result was confirmed in an in vitro assay using N-glycanase, an enzyme that releases intact N-linked glycans. Western blot analysis of MIN6 cell extracts incubated with the enzyme showed similar results to tunicamycin treatment ( image 3 b).
[0187] A chemical cross-linking test was carried out to detect whether the TMEM27 protein existed as a multimer. MIN6 cell extracts were incubated in the presence of two differ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com