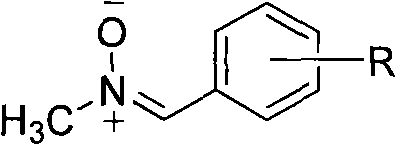
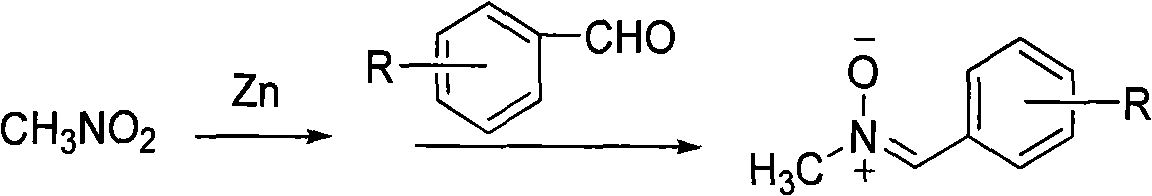
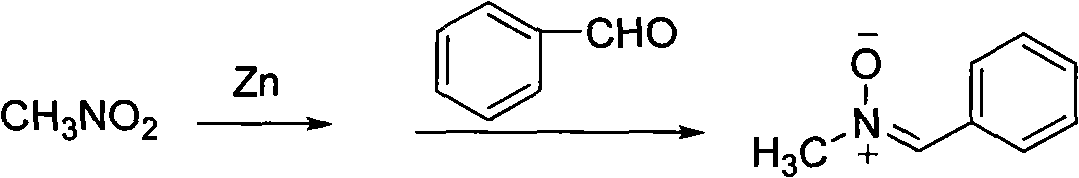
One-pot method for synthesizing N-methylnitrone compounds
A methyl nitrone and compound technology, applied in the directions of organic chemistry, oxime preparation, etc., can solve the problems of harsh reaction conditions, complex synthesis process and high synthesis cost, and achieve the effects of low synthesis cost, simple process and good product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the synthesis of α-phenyl-N-methylnitrone
[0022] Nitromethane (2.66 g, 43.6 mmol) and zinc powder (2.95 g, 45.4 mmol) were added in a 250 ml round bottom flask, then 150 ml of 95% ethanol was added as a solvent, and the mixture was placed in an ice-water bath After being cooled to below 10°C, acetic acid (5.4 grams) was added dropwise to the reaction solution, and after 3 hours of reaction, benzaldehyde (2.3 grams, 21.8 mmol) was added to the reaction solution, heated directly to 65°C, and reflux reaction 3 After 1 hour, 2.71 g of the target product was obtained by column chromatography. Yield 92.2%.
[0023] Its reaction formula is as follows:
[0024]
[0025] Spectral data: 1 HNMR (400MHz, CDCl 3 ): δ (ppm): 3.88 (s, 3H, N-CH 3 ), 7.37 (s, 1H, C-H), 7.42 (d, J=2.4Hz, 2H, Ph-H), 7.43 (d, J=0.9Hz, 1H, Ph-H), 8.22 (dd, J=3.0 , 6.6Hz, 2H, Ph-H).
Embodiment 2
[0026] Embodiment 2: the synthesis of α-(4-methoxyphenyl)-N-methylnitrone
[0027] Nitromethane (2.66 g, 43.6 mmol) and zinc powder (2.95 g, 45.4 mmol) were added in a 250 ml round bottom flask, then 150 ml of 95% ethanol was added as a solvent, and the mixture was placed in an ice-water bath After cooling to below 10°C, acetic acid (5.4 grams) was added dropwise to the reaction solution, and after 3 hours of reaction, p-methoxybenzaldehyde (2.95 grams, 21.8 mmol) was added to the reaction solution and heated directly to 65°C , refluxed for 3 hours, and separated by column chromatography to obtain 3.4 g of the target product. Yield 94.4%.
[0028] Its reaction formula is as follows:
[0029]
[0030] Spectral data of the synthesized product: 1 HNMR (400MHz, CDCl 3 ): δ (ppm): 3.85 (s, 6H, OCH 3 , NCH 3 ), 6.94(d, J=8.8Hz, 2H, Ph-H), 7.29(s, 1H, CH), 8.21(d, J=8.8Hz, Ph-H).
Embodiment 3
[0031] Embodiment 3: the synthesis of α-(2-chlorophenyl)-N-methylnitrone
[0032] Nitromethane (2.66 g, 43.6 mmol) and zinc powder (2.95 g, 45.4 mmol) were added in a 250 ml round bottom flask, then 150 ml of 95% ethanol was added as a solvent, and the mixture was placed in an ice-water bath After being cooled to below 10°C, acetic acid (5.4 g) was added dropwise to the reaction solution, and after 3 hours of reaction, 2-chlorobenzaldehyde (3.05 g, 21.8 mmol) was added to the reaction solution, and directly heated to 65°C , refluxed for 2 hours, and separated by column chromatography to obtain 3.3 g of the target product, with a yield of 89.2%.
[0033] Its reaction formula is as follows:
[0034]
[0035] Spectral data: 1 HNMR (400MHz, CDCl 3 ): δ (ppm): 3.88 (s, 3H, NCH 3 ), 7.36 (s, 1H, C-H), 8.27 (d, J=8.8Hz, 2H, Ph-H), 8.38 (d, J=8.8Hz, 2H, Ph-H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com