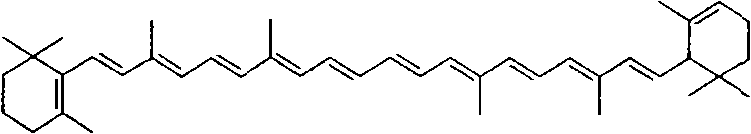
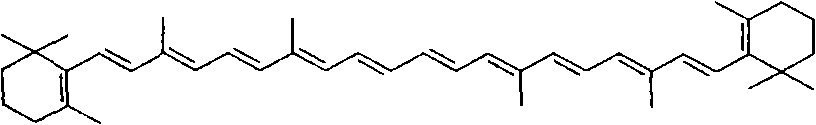
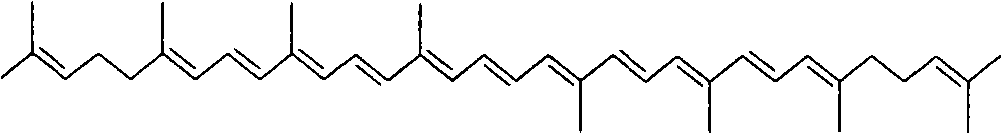
Method for synthesizing crocin glucoside
A technology of glucoside and synthesis method, which is applied in the field of synthesis of saffron glucoside, and can solve the problems such as high price of saffron glucoside
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Example 1 Synthesis of 2,7-Dimethyl-2,4,6-octatriene-1,8-dialdehyde (Compound II)
[0095] 2,7-Dimethyl-2,4,6-octatriene-1,8-dialdehyde (Compound II) has a very important position in the synthesis of conjugated polyene compounds. Through this compound, many can be synthesized Carotenoids (such as vitamin A, etc.) compounds [29-31] . In the synthesis of conjugated polyene compounds, Wittig synthesis method is one of the most important synthetic methods, and many conjugated polyene compounds are synthesized by this method [32-35] .
[0096] In the existing literature, 2,7-dimethyl-2,4,6-octatriene-1,8-dialdehyde (the synthesis of compound II starts with 1,4-butadiene or furan) The product was carried out through a four-step reaction such as Wittig, but the product obtained by the Wittig synthesis method is not easy to purify in the post-treatment and the overall yield is not high [36-38] . The product obtained by the Wittig-Horner synthesis method is easily soluble in water a...
Embodiment 2
[0146] Example 2 Synthesis of Methyl E-2-methyl-4-bromo-2-butenoate
[0147] In the existing synthetic methods [39] , The synthesis of E-2-methyl-4-halo-2-butenoate is based on α-vinyl propionate as a raw material, through halogen addition, 3,4-dihalopropionate is formed, and then removed Remove one molecule of hydrogen halide to form E-2-methyl-4-halo-2-butenoate. The reaction process is:
[0148]
[0149] There is no report on the synthesis of E-2-methyl-4-bromo-2-butenoic acid methyl ester from E-2-methyl-2-butenoic acid in the existing literature. Since E-2-methyl-2-butenoic acid is a C5 type compound, which meets the design requirements, using this compound as a raw material, first synthesize E-2-methyl-4-bromo-2-butenoic acid, and then synthesize E Methyl-2-methyl-4-bromo-2-butenoate. The synthetic route is:
[0150]
[0151] 1. Experimental part
[0152] 1.1 Instruments and reagents
[0153] 1.1.1 Instrument
[0154] Varian ANOVA-400 nuclear magnetic resonance instrumen...
Embodiment 3
[0187] Example 3 Synthesis of Dimethyl Crocetin (Compound IV)
[0188] Dimethyl Crocetin is C 20 The skeleton structure of crocetin, because dimethyl crocetin contains 7 double bonds, is a polyene compound. Based on reference to other documents [43-45] , The synthesis experiment is planned to use 2,7-dimethyl-2,4,6-octatriene-1,8-dialdehyde as C 10 Compound, E-2-methyl-4-bromo-2-butenoic acid methyl ester is C 5 The compound, using the reaction principle of Wittig-Horner, was synthesized crocetin dimethyl ester. The experimental design scheme is:
[0189]
[0190] 1. Experimental part
[0191] 1.1 Apparatus and reagents
[0192] All instruments are the same as in the second part.
[0193] 1.2 Synthesis experiment of E-2-methyl-2-butenoic acid methyl ester-4-phosphonic acid diethyl ester (Compound III)
[0194] The reaction equation for this step is as follows
[0195]
[0196] In a three-necked flask equipped with a dropping funnel, a vacuum distillation device and a thermomet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com