Methods and compositions for the treatment and prevention of infections
A subject, cyclic compound technology, applied in the direction of botany equipment and methods, drug combination, sensory disease, etc., can solve the problems of stability and effectiveness, unused potential of antibacterial agents, underutilized sodium perborate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
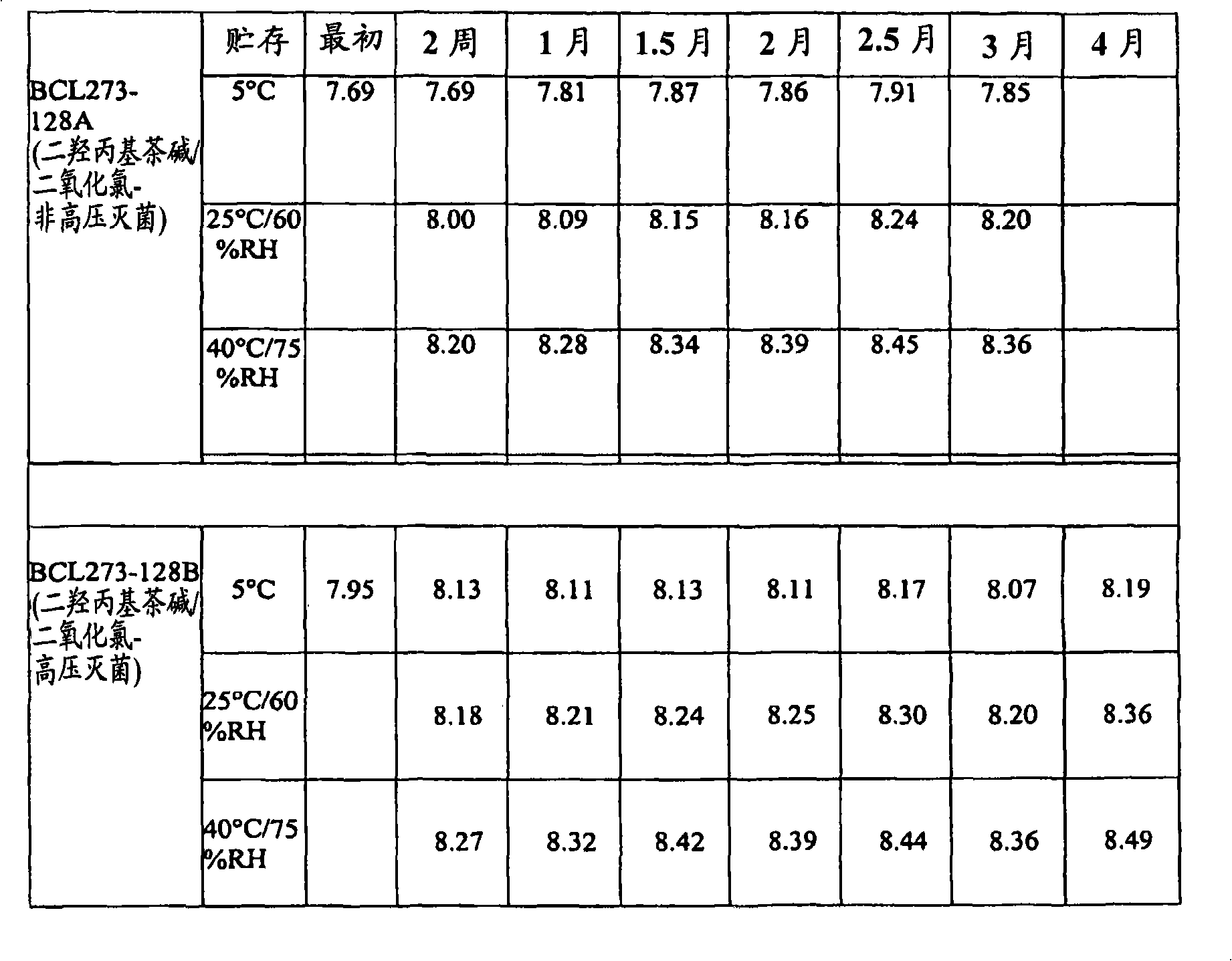
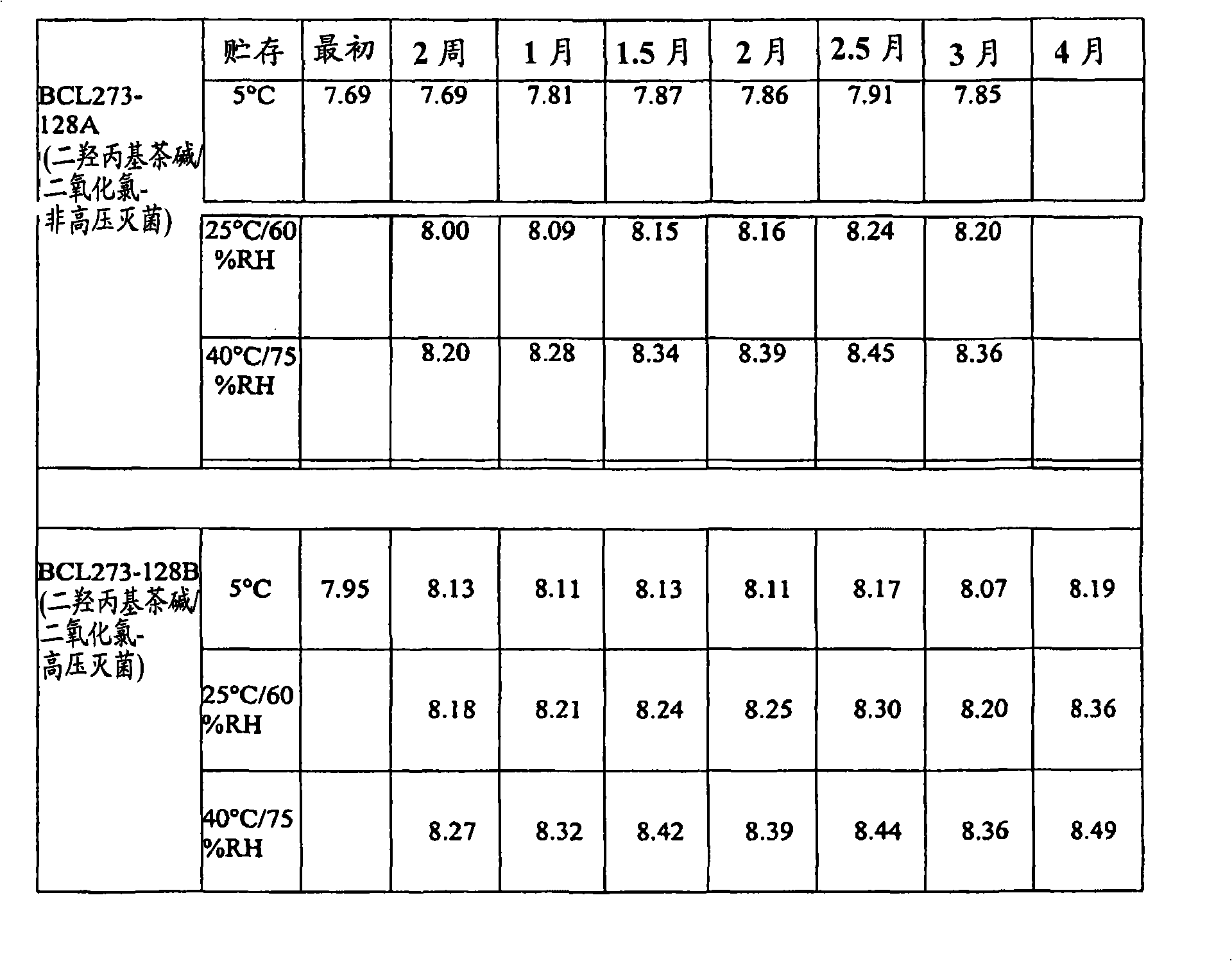
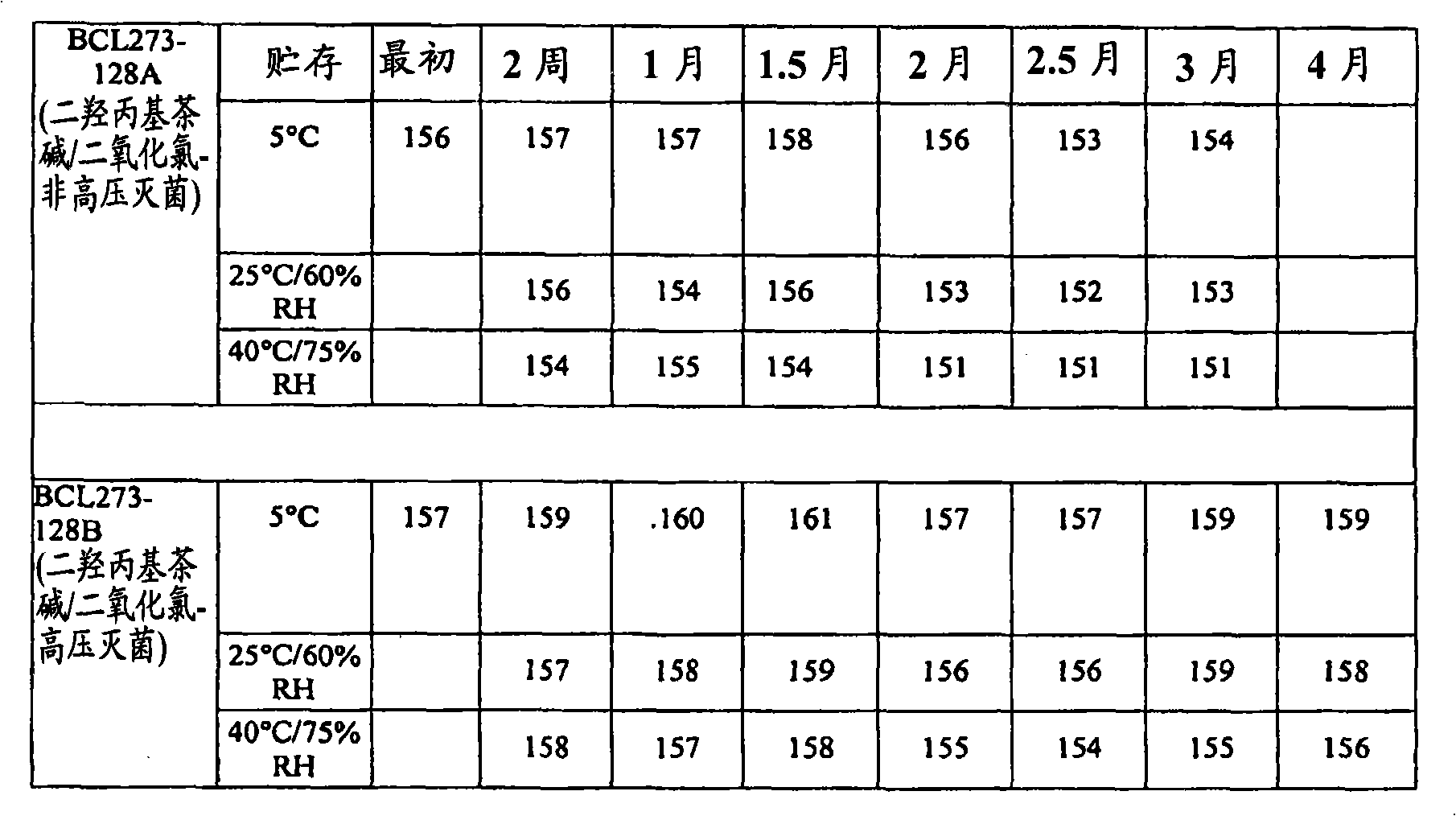
[0072] A study was conducted to evaluate the stability of three different eye care formulations containing chlorine dioxide. Base formulations were prepared with 0.005% chlorine dioxide alone, or with 0.005% chlorine dioxide and 2.5% dyphylline. This study incorporates the evaluation of each formulation with and without autoclave sterilization during manufacture.
[0073] The analysis was conducted under three storage conditions: 5°C, 25°C / 60% Relative Humidity (RH) and 40°C / 75%RH, tested and analyzed by the USP Antimicrobial Effectiveness (Preservative Efficacy) over a three-month time frame Test both compositions. Analytical tests for chlorine dioxide content, pH and osmolality were performed every two weeks for three months. Antimicrobial effectiveness of formulations was evaluated on a monthly basis based on acceptance criteria for Class 1 articles in initial antimicrobial effectiveness testing.
[0074] Analytical testing of the dyphylline / chlorine dioxide samples show...
Embodiment 5
[0077] During the conduct of the initial study, a confirmatory study was performed to confirm the results of the antimicrobial effectiveness of the autoclaved dyphylline / chlorine dioxide samples. In addition to this sample, an autoclaved chlorine dioxide formulation and formulation was prepared based on Example 5 of US Patent 6,024,954 (consistent with Refresh). Using the USP antimicrobial effectiveness test, all three formulations containing dyphylline / chlorine dioxide and the formulation based on Example 5 of US Patent 6,024,954 (consistent with Refresh), and the formulation containing chlorine dioxide alone were tested after 28 days. The sample failed again on day 7.
[0078] introduce
[0079] For stability evaluation, formulations were prepared with the following variations:
[0080] Table 1: Chlorine Dioxide Formulations
[0081] Chemicals
percentage
CMC
0.25%
0.22218%
0.07315%
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com