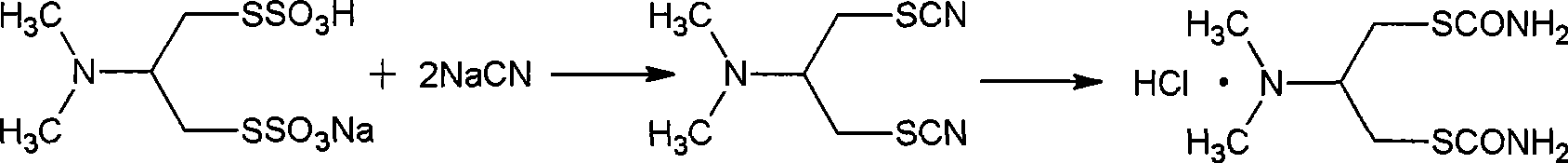
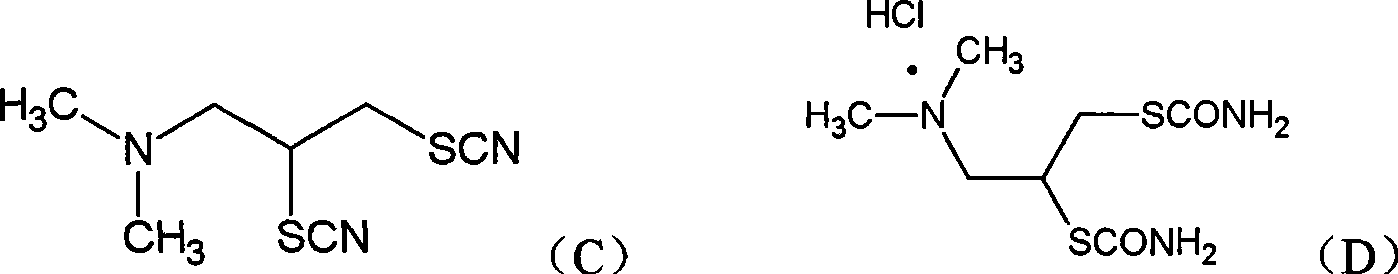Preparation method of cartap hydrochloride intermediate, i.e., 2-N, N-dimethyl-1, 3-dithio-cyano propane
A technology of dithiocyanopropane and dimethyl, which is applied in the field of thiocyanate intermediate 2-N, can solve the problems of low thiocyanate yield, high processing cost, poor working environment and the like, and achieves convenient process implementation, The effect of high yield of normal isomer and less amount of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Put 400g of n-butanol, 115g of sodium thiocyanate, and 110g of 1-N,N-dimethyl-2,3-dichloropropylamine with a content of 95% into the reaction flask. Any value can be selected within the range of 45~85℃, including the end value), heat preservation reaction for 5 hours, the ratio of thiocyanide normal and isomer: normal structure 51%, isomer 48%, filter out the chlorination Sodium inorganic salt, the filtrate cooled to precipitate crystals, filtered to obtain thiocyanide isomer solid crystals (normal form 5%, isomer 94%), dried 57g, the filtrate was frozen and crystallized, filtered to obtain thiocyanide normal form ( The normal form is 97%, the isomer is 2%), 54g is dry, and the yield of the normal form of thiocyanide is 39%.
Embodiment 2
[0023] Example 2: Put 400g of n-butanol into the reaction flask, 57g of the isomer obtained in Example 1, 58g of sodium thiocyanate, 55g of 95% 1-N,N-dimethyl-2,3-dichloride Propylamine, heat up 45~85℃ (any value can be selected within the range of 45~85℃, including the end value), heat preservation reaction for 5 hours, the ratio of normal and isomer of thiocyanide, normal body 51%, isomer 47 %, filtered to remove the sodium chloride inorganic salt generated, the filtrate was cooled to precipitate crystals, and filtered to obtain solid crystals of thiocyanide isomers (normal structure 6%, isomer 93%), dried 58g, and the filtrate was frozen and crystallized. The normal form of thiocyanide (98% of normal form and 1% of isomer) was obtained by filtration, which was 53 g in dry form. The yield of normal form of thiocyanide was 77%.
Embodiment 3
[0024] Example 3: Put 500g of acetone into the reaction flask, 58g of the isomer obtained in Example 2, 58g of sodium thiocyanate, 55g of 1-N,N-dimethyl-2,3-dichloropropylamine with a content of 95%, The temperature is increased to 45~70℃ (any value can be selected within the range of 45~70℃ and the end value is included), and the reaction is kept for 5 hours. The ratio of normal thiocyanide to isomer is 50% for normal isomer and 48% for isomer. The inorganic salt of sodium chloride was filtered off, the filtrate was cooled to precipitate crystals, and the solid crystals of thiocyanide isomers were obtained by filtration (normal form 5%, isomer 94%), which was dried 58g, the filtrate was frozen and crystallized, and filtered to obtain The normal form of thiocyanide (98% of normal form, 1% of isomer), 52g in dry form, the yield of normal form of thiocyanide is 76%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com