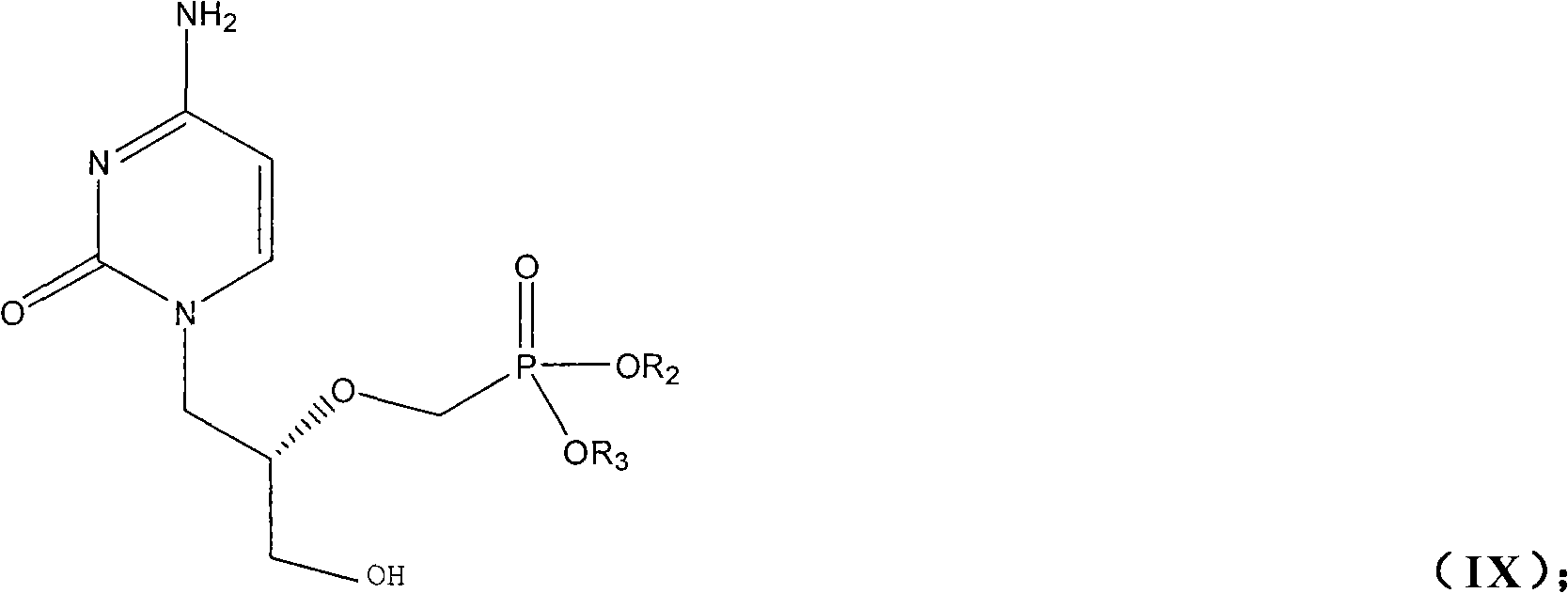
Preparation method of Cidofovir and intermediate thereof
A compound, selected technology, applied in the direction of chemical instruments and methods, compounds of group 5/15 elements of the periodic table, organic chemistry, etc., can solve the problem of low synthesis yield, synthetic process, unsuitable for industrialization, multiple side reactions product and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Embodiment 1: the preparation of cidofovir
[0084] Preparation of (S)-1-benzyloxy-2,3-epoxypropane
[0085] Add 21.6g (0.2mol) of benzyl alcohol into 120mL of 50% sodium hydroxide, cool down to 0°C, add 74g (0.8mol) (R)-epichlorohydrin dropwise over 30min, and then react below 10°C 20h, gas spectrum tracking reaction ended. The reactant was poured into 70 mL of ice water. They were extracted with dichloroethane (2×70 mL), combined, washed once with 50 mL of water, once with 50 mL of saturated NaCl solution, and dried over anhydrous magnesium sulfate. Precipitation gave 26.7 g of anhydrous liquid. The yield is 83.5%, and the content is greater than 97%.
[0086] N 4 - Preparation of Benzoylcytosine
[0087]3.0 g of cytosine was added to 300 mL of pyridine, and 37.5 mL of benzoyl chloride was added dropwise. Stir at room temperature and add dropwise for half an hour. Stir for another hour. 2N hydrochloric acid solution was added dropwise and stirred at room temp...
Embodiment 2
[0099] Example 2(S)-N 1 -[(3-Hydroxy-2-phosphodiethylmethoxy)glycerol]-N 4 - Preparation of cytosine
[0100] Preparation of (S)-trityloxy-2,3-epoxypropane
[0101] Add 42.3g (0.2mol) of trityl alcohol into 150mL of 50% sodium hydroxide, cool down to 0°C, add 74g (0.8mol) (R)-epibromohydrin dropwise over 30min, and then lower the temperature below 30°C After 20 hours of reaction, the gas spectrum tracking reaction ended. The reactant was poured into 70 mL of ice water. They were extracted with dichloroethane (2×70 mL), combined, washed once with 50 mL of water, once with 50 mL of saturated NaCl solution, and dried over anhydrous magnesium sulfate. Precipitation gave 32.6 g of anhydrous liquid. The yield is 76.5%, and the content is greater than 92%.
[0102] N 4 - Preparation of Benzoylcytosine
[0103] 5.0 g of cytosine was added to 300 mL of toluene, and 37.5 mL of benzoyl bromide was added dropwise. Stir at room temperature, add dropwise for half an hour, and the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com