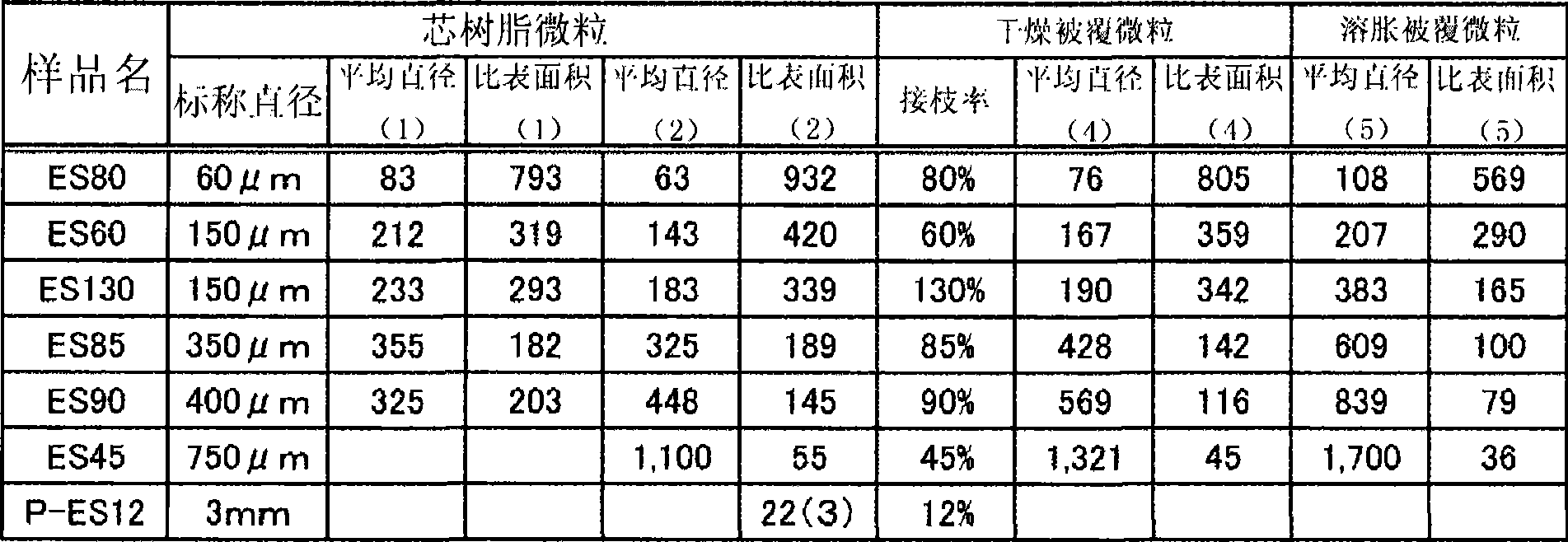
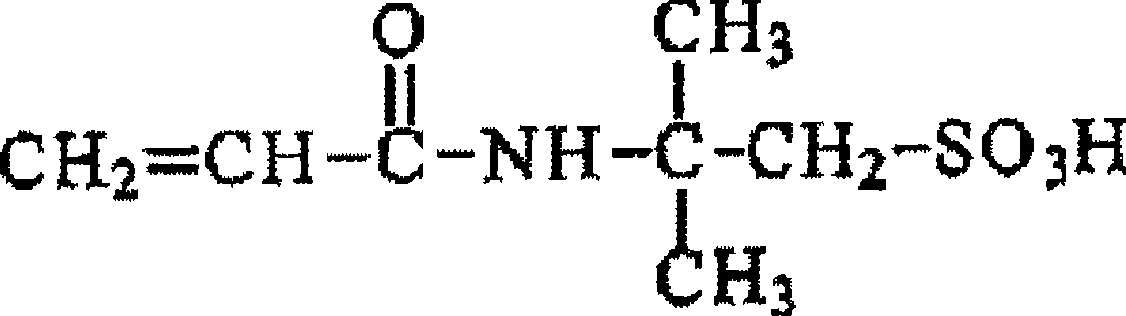Coated particles, process for production thereof, cation adsorbents, and water treatment system
A manufacturing method and cation technology, applied in the direction of ion exchange water/sewage treatment, ion exchange treatment device, cation exchange, etc., can solve the problems of chlorine odor hazard, generation of trihalomethane, excess input, etc., and achieve high adsorption capacity, The effect of fast regeneration speed and fast adsorption speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
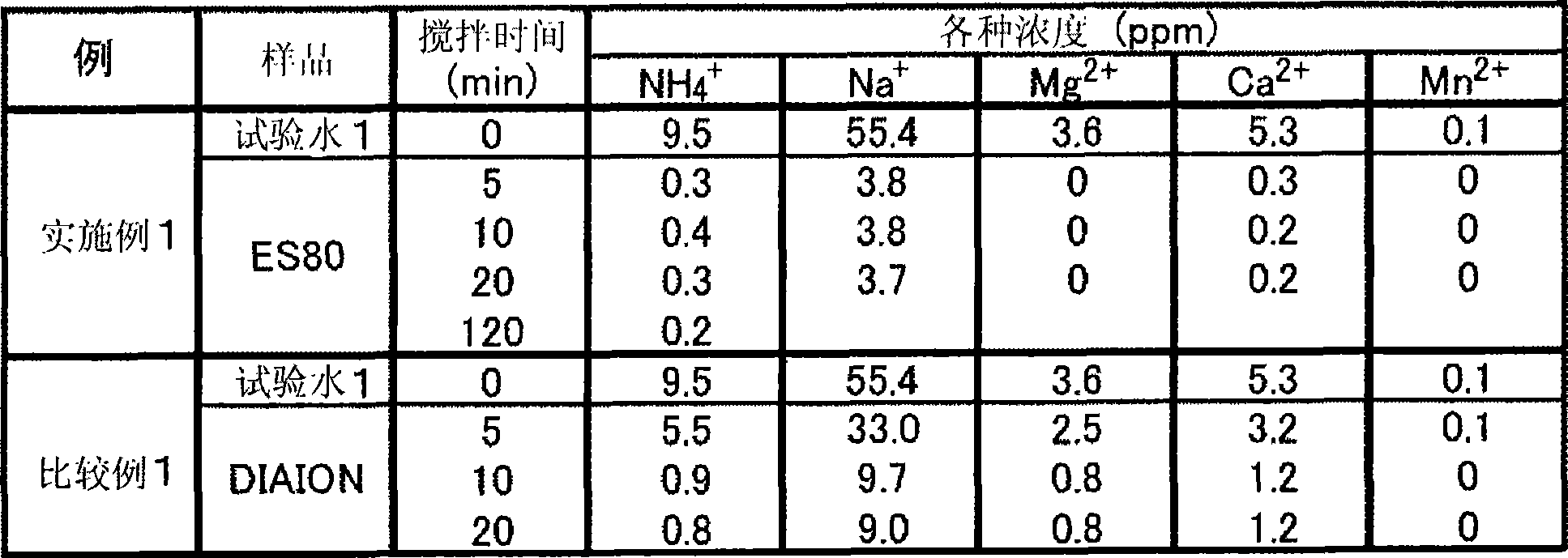
Embodiment 1 and comparative example 1
[0107] [Table 4]
[0108]
[0109] From the above examples, it can be seen that the ammonium ion reaches the adsorption equilibrium at the target 1 ppm or less within 5 minutes. In addition, in this case, the other ions contained in the test water also reached the saturation concentration or the detection limit or less within 5 minutes. On the other hand, in Comparative Example 1, it took 10 minutes or more to reach adsorption equilibrium.
Embodiment 2
[0111] The sorbent sample was ES80 used in Example 1. The test water was the same as the ammonia water used in Example 1. Measurement of NH in treated water after 1 minute and 3 minutes after contact with cationic adsorbent 4 + concentration of ions.
[0112] Furthermore, from this Example 2, it was confirmed that the adsorbent of the present invention reached adsorption equilibrium within 1 minute.
[0113] [table 5]
[0114] Ion species: NH 4 + The initial concentration 1 minute later 3 minutes later Ion concentration (ppm) 9.5 0.5 0.5
Embodiment 3 and comparative example 2
[0116] Using the test water 2 shown in Table 6, an adsorption test was performed, and the ion concentration over time was measured.
[0117] [Table 6]
[0118]
[0119] It can be seen from Example 3 that the adsorbent of the present invention reaches adsorption equilibrium within 3 minutes when the grafting rate is low. In contrast, in Comparative Example 2 using a zeolite-based adsorbent, it took 10 minutes or more to reach equilibrium.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com