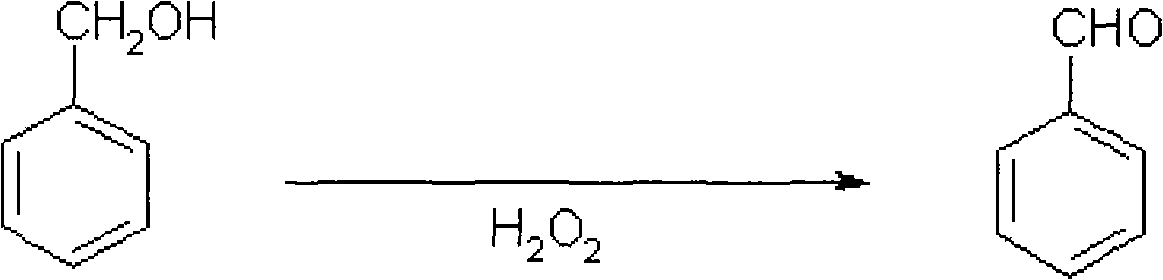
Catalytic synthesizing method of benzaldehyde
A synthesis method and technology of benzaldehyde, which are applied in chemical instruments and methods, oxidation preparation of carbonyl compounds, catalysts for physical/chemical processes, etc., can solve the problems of difficult post-processing of the reaction system, difficult separation of catalysts and products, and environmental pollution by organic solvents. , to achieve the effect of improving economic and social benefits, low production cost and short response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0007] Specific embodiment one: the catalytic synthesis method of benzaldehyde in the present embodiment is realized according to the following steps: one, 3.5 ~ 13mL of benzyl alcohol, ethanol, propanol, butanol, amyl alcohol, isoamyl alcohol or isobutanol are added to three Neck flask, then add 0.1-0.5g of catalyst to obtain mixture A; 2. Heat mixture A from room temperature to 80-120°C, then gradually drop hydrogen peroxide with a mass fraction of 30% and a volume of 20-30mL Add it to mixture A and stir for 4-8 hours, so that the molar ratio of hydrogen peroxide to benzyl alcohol is 1.5-8.5:1 to obtain mixture B; 3. Add 30-50 mL of benzene to mixture B to extract and separate the layers, discard The upper layer was removed, and then the liquid phase in the lower layer was distilled by rotary evaporation, and the solid phase was then washed once with 20 mL of benzene, 20 mL of water and 50 mL of ethyl acetate, and then filtered and dried to obtain benzene Formaldehyde; where...
specific Embodiment approach 2
[0011] Embodiment 2: The difference between this embodiment and Embodiment 1 is that the amount of benzyl alcohol, ethanol, propanol, butanol, amyl alcohol, isoamyl alcohol or isobutanol in step 1 is 4.5-12 mL. Other steps and parameters are the same as those in Embodiment 1.
specific Embodiment approach 3
[0012] Embodiment 3: This embodiment differs from Embodiment 1 in that the amount of benzyl alcohol, ethanol, propanol, butanol, amyl alcohol, isoamyl alcohol or isobutanol in step 1 is 5.5-10 mL. Other steps and parameters are the same as those in Embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com