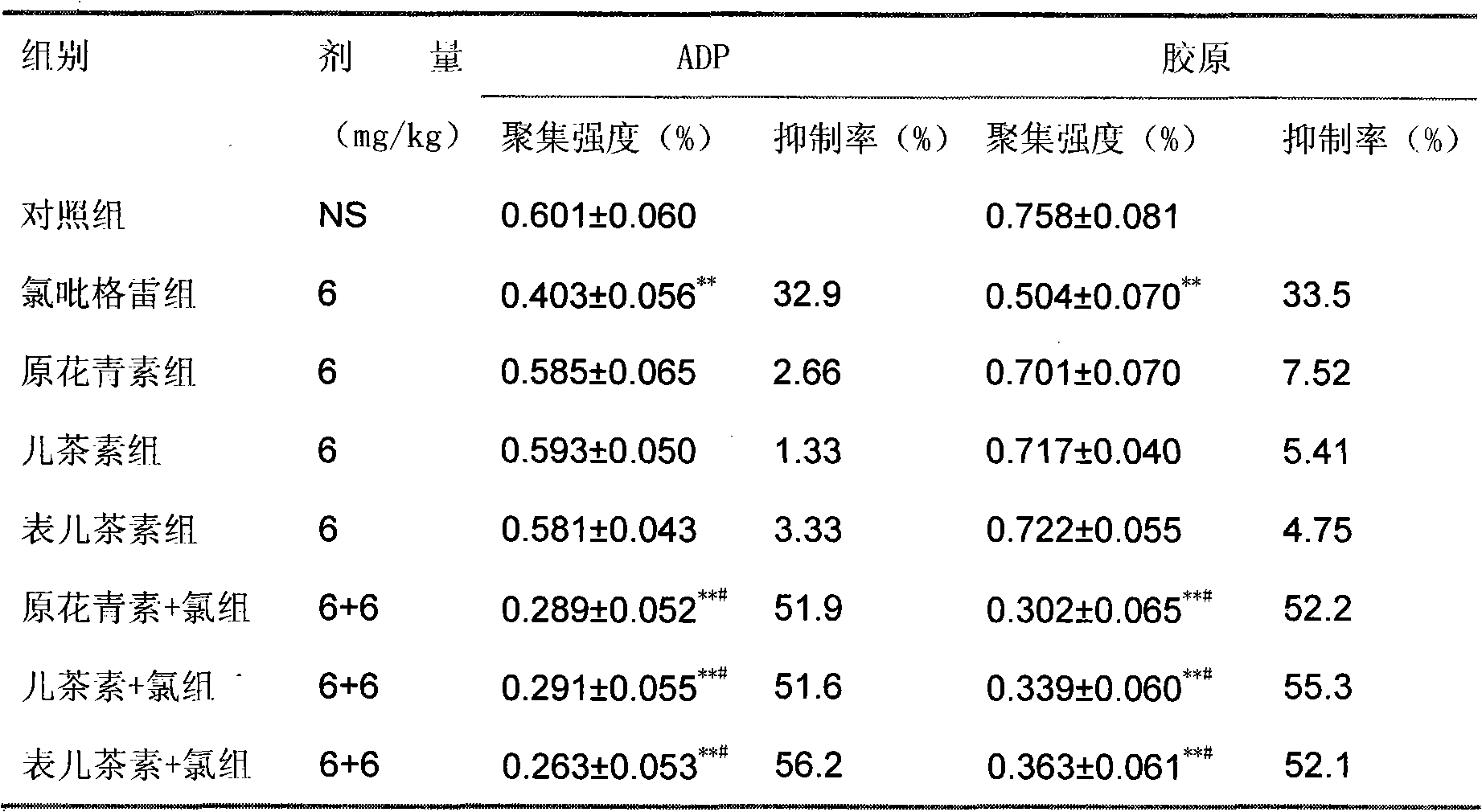
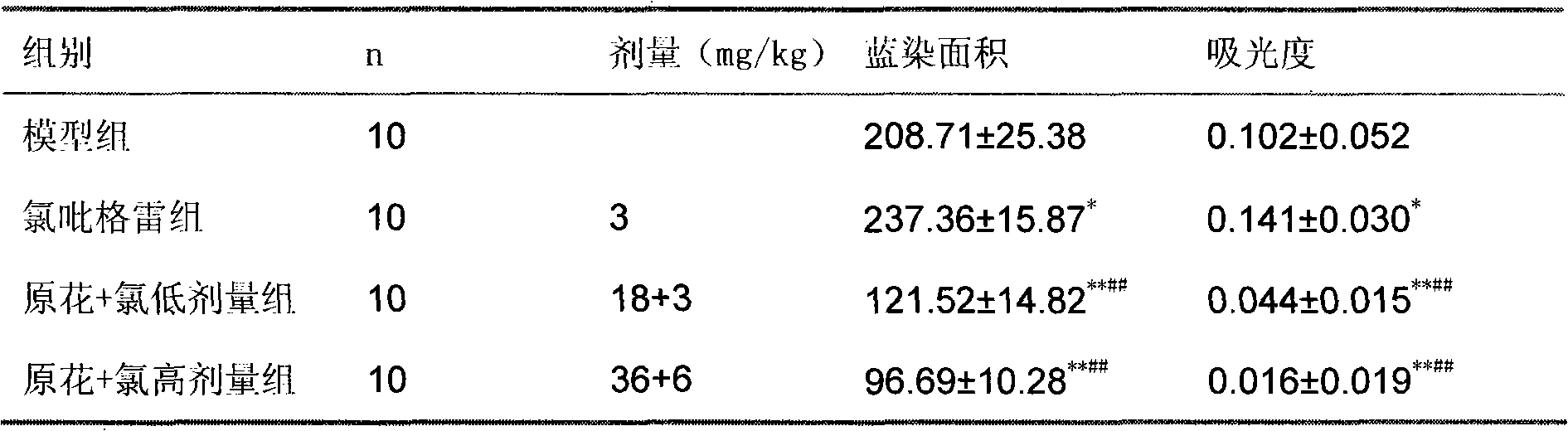
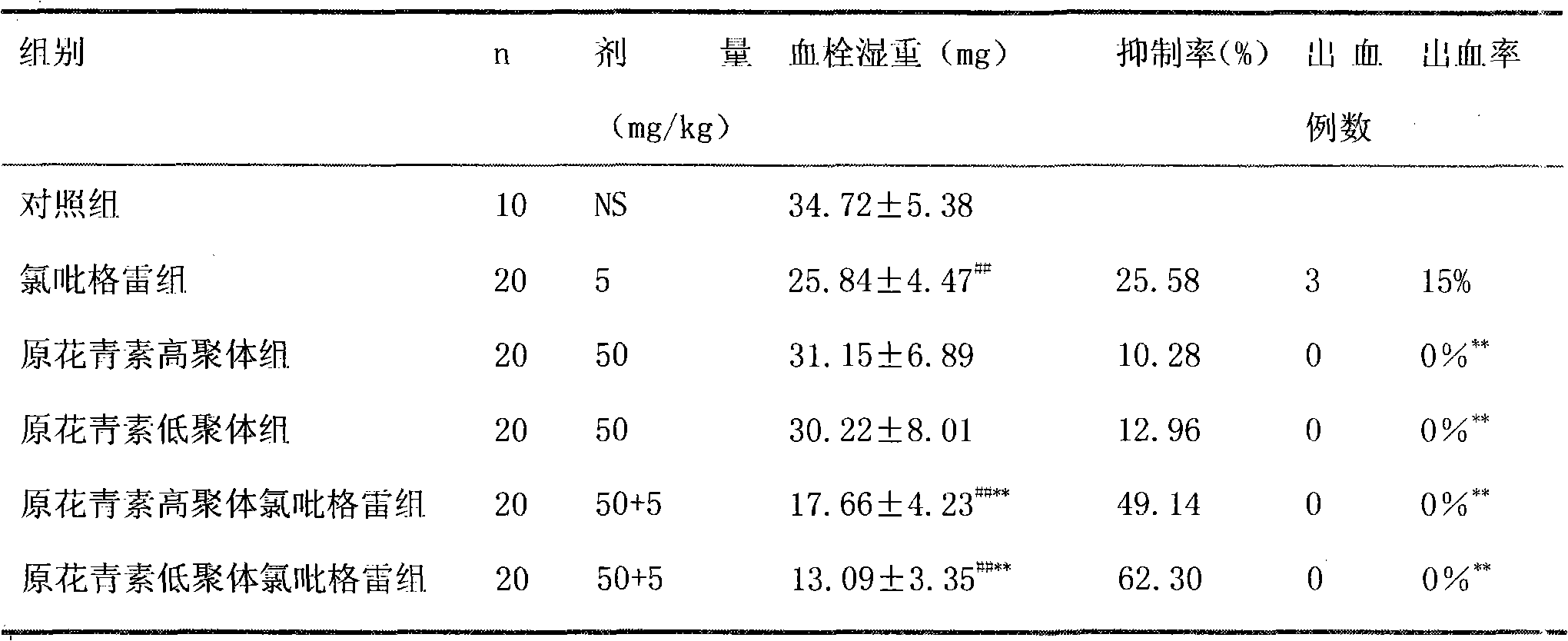
Pharmaceutical composition containing clopidogrel
A clopidogrel and composition technology, applied in the directions of drug combinations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problems of increased bleeding risk, rebound of cardiovascular events, easy to cause bleeding, etc., and achieve the bleeding rate Reduce, enhance anticoagulant activity, inhibit platelet aggregation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Embodiment 1 common tablet
[0013] Proanthocyanidins 25g
[0014] Clopidogrel Bisulfate 25g
[0015] Microcrystalline Cellulose 250g
[0016] Lactose 20g
[0017] 10% starch slurry appropriate amount
[0018] Magnesium Stearate 1.5g
[0019] Preparation process: Weigh the prescribed amount of proanthocyanidins, clopidogrel bisulfate, microcrystalline cellulose, and lactose and mix evenly. In addition, add an appropriate amount of 10% starch slurry to the mixed powder, mix evenly, make soft material, pass through a 18-mesh nylon sieve to make wet granules, and dry at about 60°C. The moisture content of the dry granules should be controlled below 1.5%. Sieve through a 20-mesh sieve, mix with magnesium stearate, and press into tablets.
Embodiment 2
[0020] Embodiment 2 common tablet
[0021] Catechin 5g
[0022] Clopidogrel Hydrochloride 25g
[0023] Starch 400g
[0024] Dextrin 20g
[0025] 50% ethanol appropriate amount
[0026] Magnesium Stearate 2.0g
[0027] Preparation process: Weigh the prescribed amount of catechin, clopidogrel hydrochloride, starch, and dextrin and mix evenly. In addition, an appropriate amount of 50% ethanol is added to the mixed powder, mixed evenly, made into soft material, passed through a 18-mesh nylon sieve to make wet granules, and dried at about 60°C. The moisture content of the dry granules should be controlled below 1.5%. Sieve through a 20-mesh sieve, mix with magnesium stearate, and press into tablets.
Embodiment 3
[0028] Embodiment 3 Dispersible tablets
[0029] Epicatechin 600g
[0030] Clopidogrel 15g
[0031] Croscarmellose Sodium 10g
[0032]Microcrystalline Cellulose 500g
[0033] Polyvinylpyrrolidone 50g
[0034] 5% PVP 60% ethanol solution appropriate amount
[0035] Micronized silica gel 5g
[0036] Preparation process: Weigh epicatechin and clopidogrel according to the prescription, use microcrystalline cellulose as filler, croscarmellose sodium and polyvinylpyrrolidone as disintegrants, 5% PVP 60% ethanol solution It is used as binder, and micronized silica gel is used as flow aid. It is granulated in one step in a fluidized bed, and then compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com