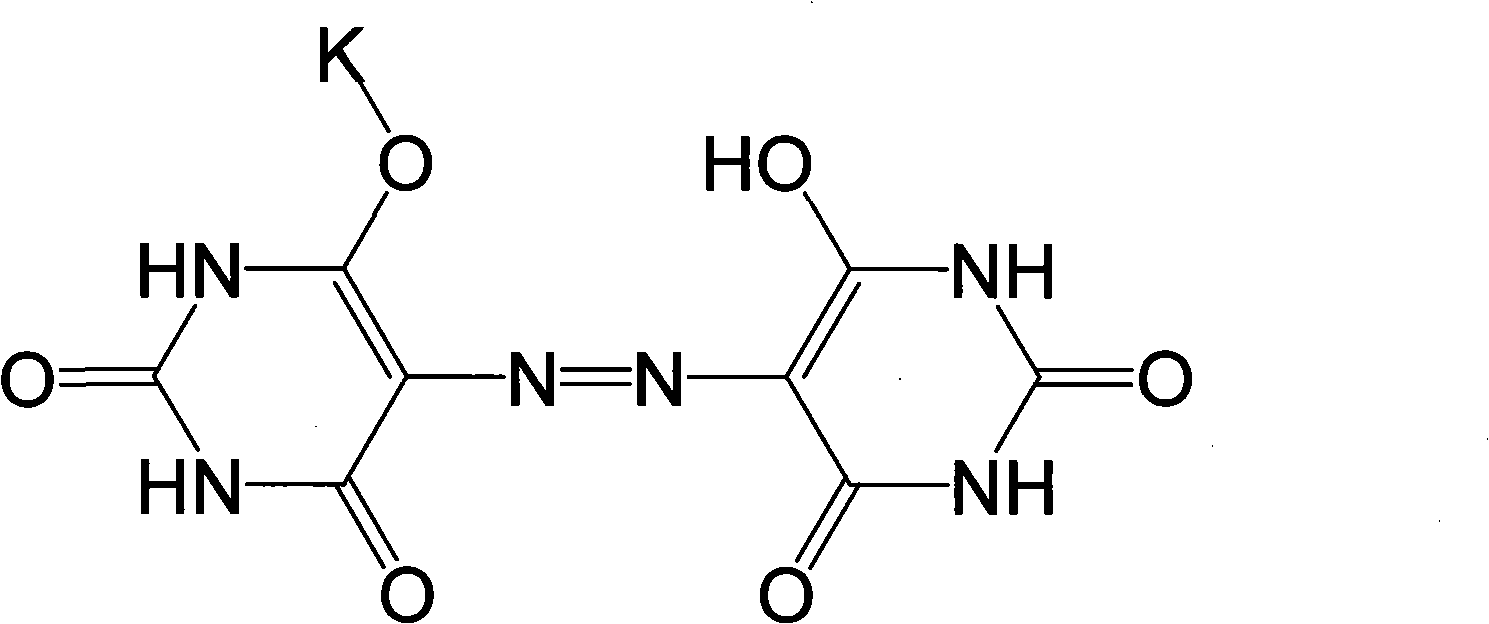
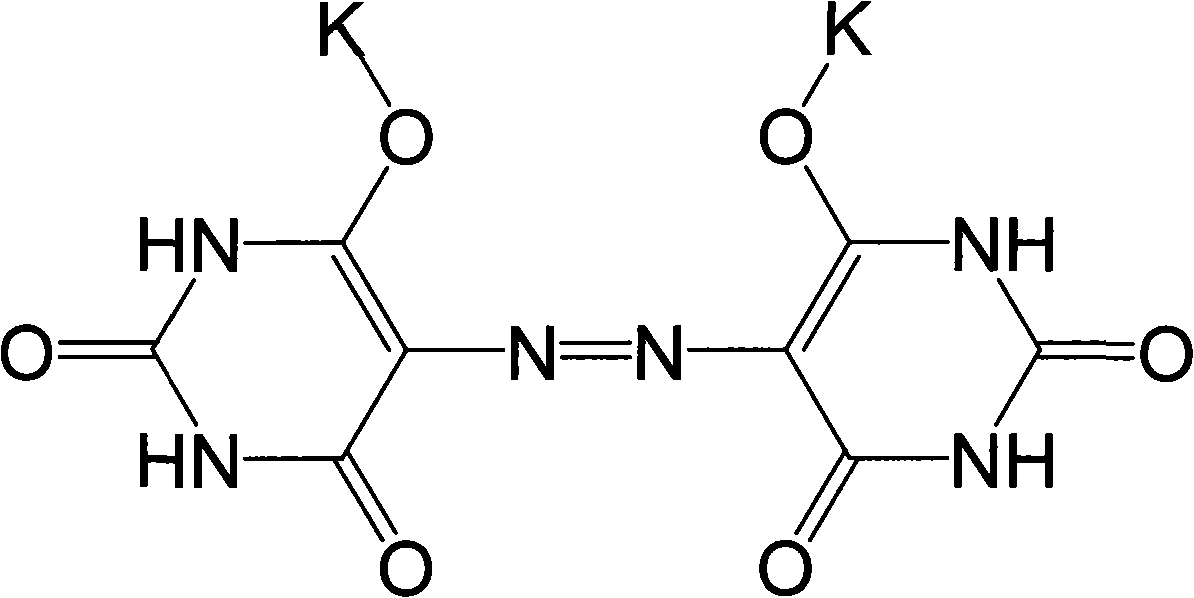
Method for preparing pigment yellow 150 with salt mixture of kalium azoic barbiturate and natrium azoic barbiturate
A technology of potassium azobarbiturate and barbituric acid, which is applied in the directions of azo dyes, complex metal compounds of azo dyes, chemical instruments and methods, etc., can solve the changes in fluidity, weather resistance and solvent resistance. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
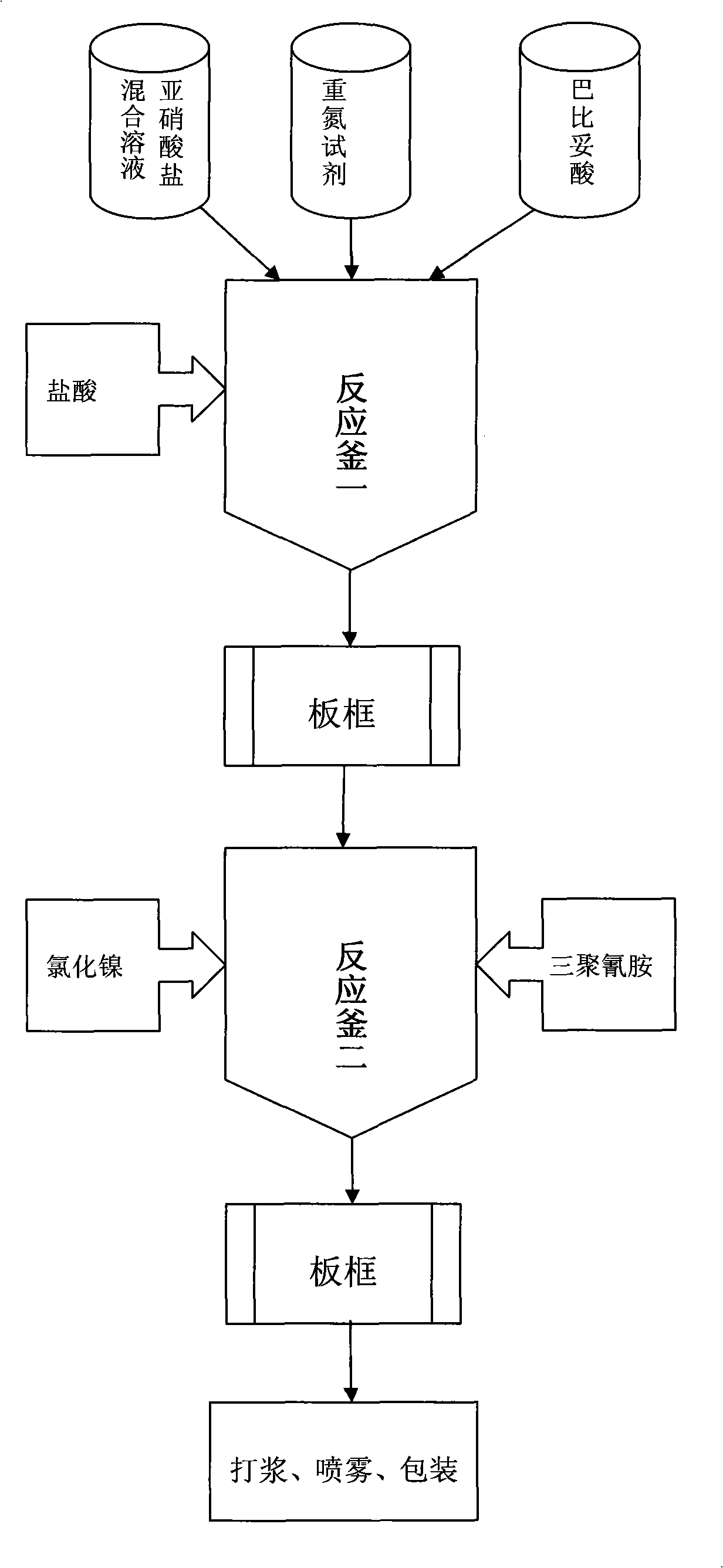
[0029] 1. Prepare potassium and sodium azobarbiturate mixed salt with aminoguanidine carbonate, and then prepare nickel barbiturate complex pigment; then apply the present invention to prepare Pigment Yellow 150.
example 1
[0030] Example 1: The molar ratio of sodium nitrite to potassium nitrite is 8:2
[0031] (1) Dissolve 40 grams of aminoguanidine carbonate in 240 grams of distilled water and stir for 10 minutes.
[0032] (2) Slowly add 82 grams of 31% hydrochloric acid and stir for 5 minutes.
[0033] (3) Cool the system to -10°C with 280 grams of ice, slowly add a mixed solution of sodium nitrite and potassium nitrite dropwise within one hour (16.39 grams of sodium nitrite and 5.05 grams of potassium nitrite are dissolved in 45 grams of distilled water ), kept at 15°C for 15 minutes, and a small amount of sulfamic acid was added to remove excess nitrite ions.
[0034] (4) Add 79.1 grams of barbituric acid and stir for two hours.
[0035] (5) Adjust the pH value of the system to 2.5 with 25% potassium hydroxide solution, and stir for 30 minutes.
[0036] (6) Adjust the pH value of the system to 4.8 with 25% potassium hydroxide solution, and stir for 30 minutes.
[0037] (7) Raise the temp...
example 2
[0040] Example 2: The molar ratio of sodium nitrite to potassium nitrite is 7:3
[0041] (1) Dissolve 40 grams of aminoguanidine carbonate in 240 grams of distilled water and stir for 10 minutes.
[0042] (2) Slowly add 82 grams of 31% hydrochloric acid and stir for 5 minutes.
[0043] (3) Cool the system to -10°C with 280 grams of ice, and slowly add a mixed solution of sodium nitrite and potassium nitrite (14.35 grams of sodium nitrite and 7.57 grams of potassium nitrite dissolved in 45 grams of distilled water) within one hour. ), kept at 15°C for 15 minutes, and a small amount of sulfamic acid was added to remove excess nitrite ions.
[0044] (4) Add 79.1 grams of barbituric acid and stir for two hours.
[0045] (5) Adjust the pH value of the system to 2.5 with 25% potassium hydroxide solution, and stir for 30 minutes.
[0046] (6) Adjust the pH value of the system to 4.8 with 25% potassium hydroxide solution, and stir for 30 minutes.
[0047] (7) Raise the temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com