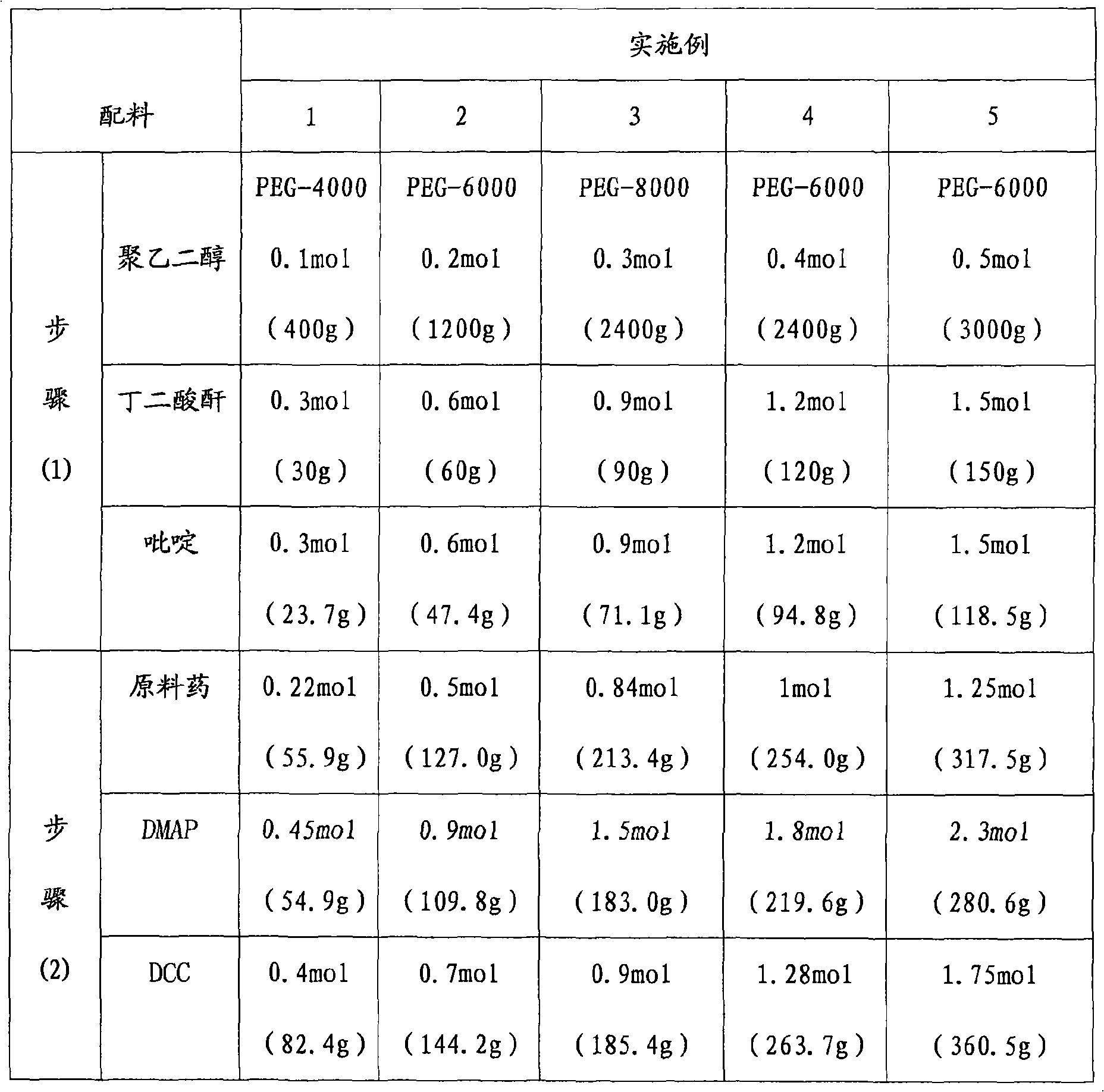
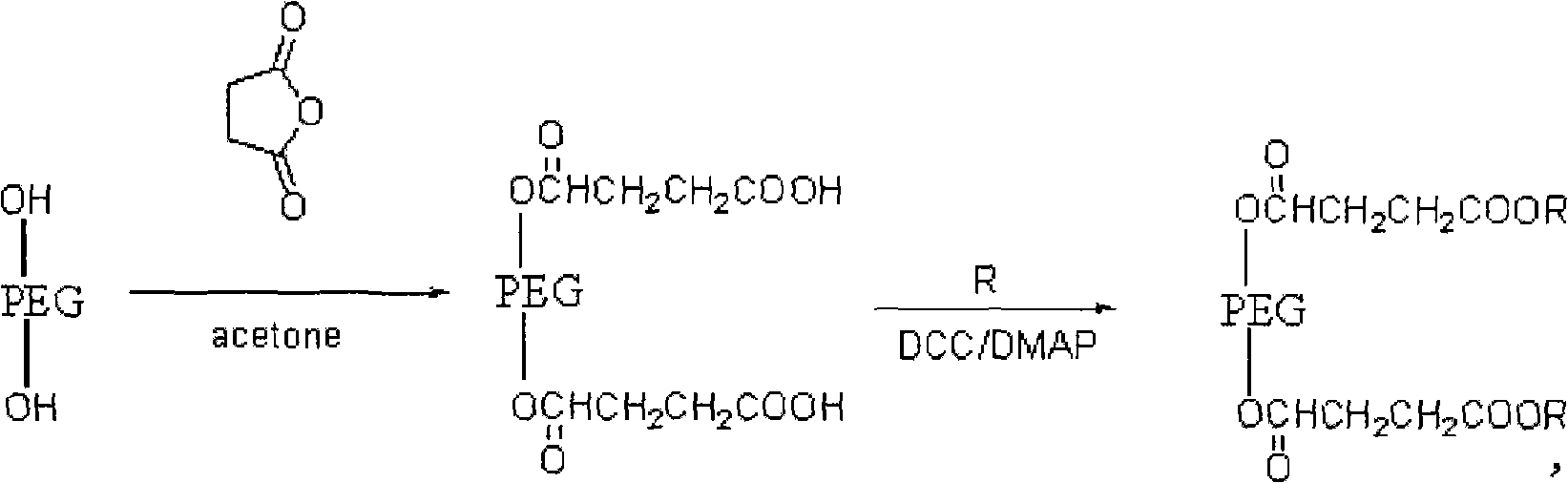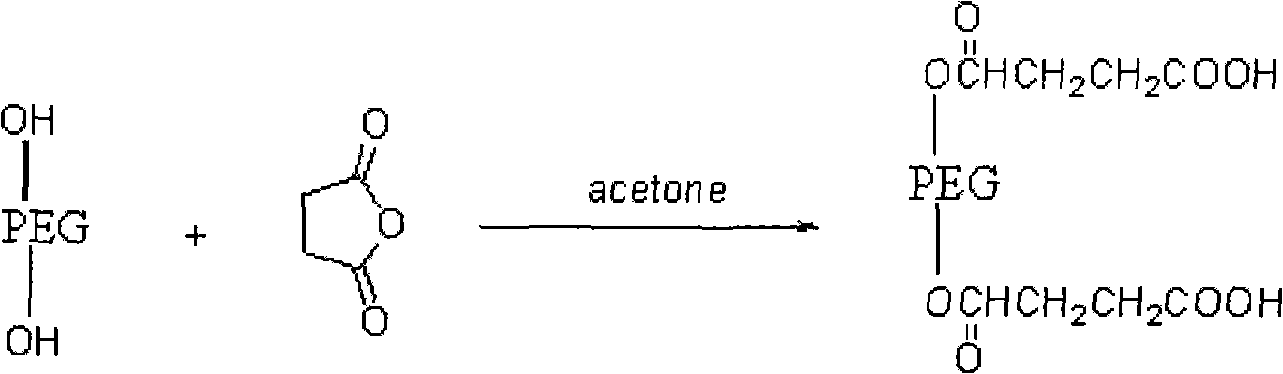3, 5-dihydroxy-4-isopropyl diphenyl ethylene-butanedioic anhydride-polyoxyethylene compound and synthetic method thereof
A technology of isopropyl stilbene and hydroxy stilbene, which is applied in the field of prodrugs of antifungal drugs, can solve the problems of inability to maintain effective blood drug concentration, low absolute bioavailability, short biological half-life, etc. Bioavailability, good water solubility, effect of altering distribution in the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021]
[0022] The synthetic route of embodiment 1-embodiment 5 is as follows:
[0023]
[0024] in
[0025] The synthetic method of embodiment 1-embodiment 5 is carried out according to the following step sequence respectively:
[0026] (1) Preparation of polyethylene glycol succinate
[0027] Take dry polyethylene glycol and succinic anhydride with a molar ratio of 1:3, mix them, dissolve them in acetone, add pyridine with the same molar number as succinic anhydride, heat to reflux; evaporate the solvent, add saturated NaHCO 3 solution, extracted with ethyl acetate, adjusted to pH=2.8-3.2 with dilute hydrochloric acid; extracted with chloroform, dried, concentrated under reduced pressure, added anhydrous ether, and precipitated a white solid A, polyethylene glycol succinate;
[0028] This step reaction formula is as follows:
[0029]
[0030] (2) Preparation of polyethylene glycol-succinic acid-bis(4-isopropyl-5-hydroxyl stilbene-3-phenol ester)
[0031] Take...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com