Method for synthesizing 2-methyl-1,4-naphthaquinone by taking ionic liquid as catalyst
An ionic liquid and catalyst technology, applied in the field of synthesizing 2-methyl-1, can solve the problems of chromium-containing wastewater and serious environmental pollution, and achieve the effect of fast reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Preparation of the first step ionic liquid
[0014] Stir equimolar pyridine (15.82g) and 1,4-butane sultone (27.23g) at room temperature for 3 days to obtain a white solid, wash with ethyl acetate to obtain a white solid ylide; then weigh equimolar The ylide (4.31g) and phosphoric acid (85%, 2.31g) were stirred and reacted at 80°C for 6h, and then rotary evaporated at 80°C for 4h to obtain the ionic liquid [SO 3 H-BPy][H 2 PO 4 ].
[0015] Synthesis of the second step 2-methyl-1,4-naphthoquinone
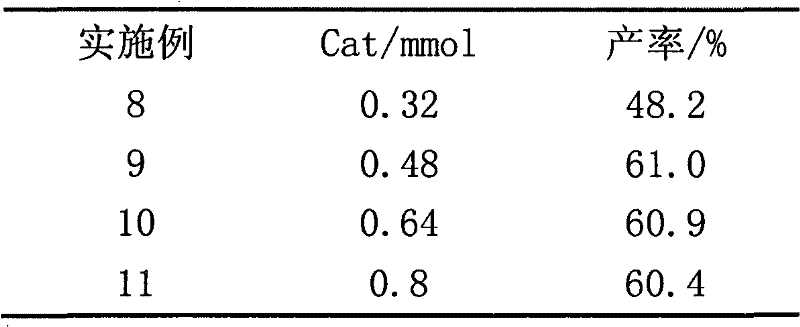
[0016] Add 0.05mol 2-methylnaphthalene, 0.25mol glacial acetic acid and 0.48mmol ionic liquid [SO 3 H-BPy][H 2 PO 4 ], when the above reactants were heated to 90°C, 20ml H 2 o 2 (30%, H 2 o 2 0.2 mol) was added dropwise in 20 minutes, and the temperature was maintained for 2 hours. During the reaction, samples were taken regularly for GC-MS qualitative and GC quantitative analysis, and the yield was 61%.
Embodiment 2-4
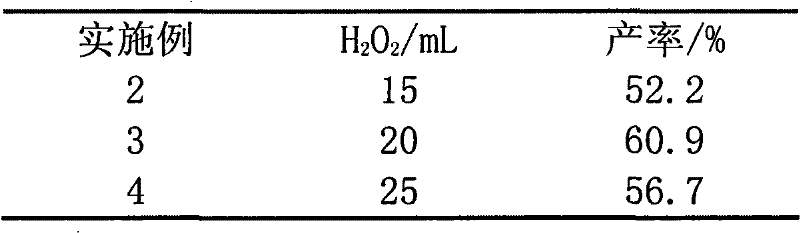
[0018] Except that following and catalyst (0.64mmol) are different, all the other are identical with embodiment 1, H 2 o 2 Dosage according to Table 1.
[0019] Table 1
[0020]
Embodiment 5-7
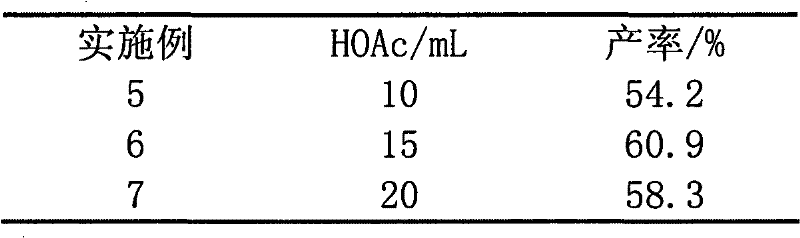
[0022] Except that following and catalyst (0.64mmol) are different, all the other are identical with embodiment 1, and HAOc consumption is by table 2 consumption.
[0023] Table 2
[0024]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com