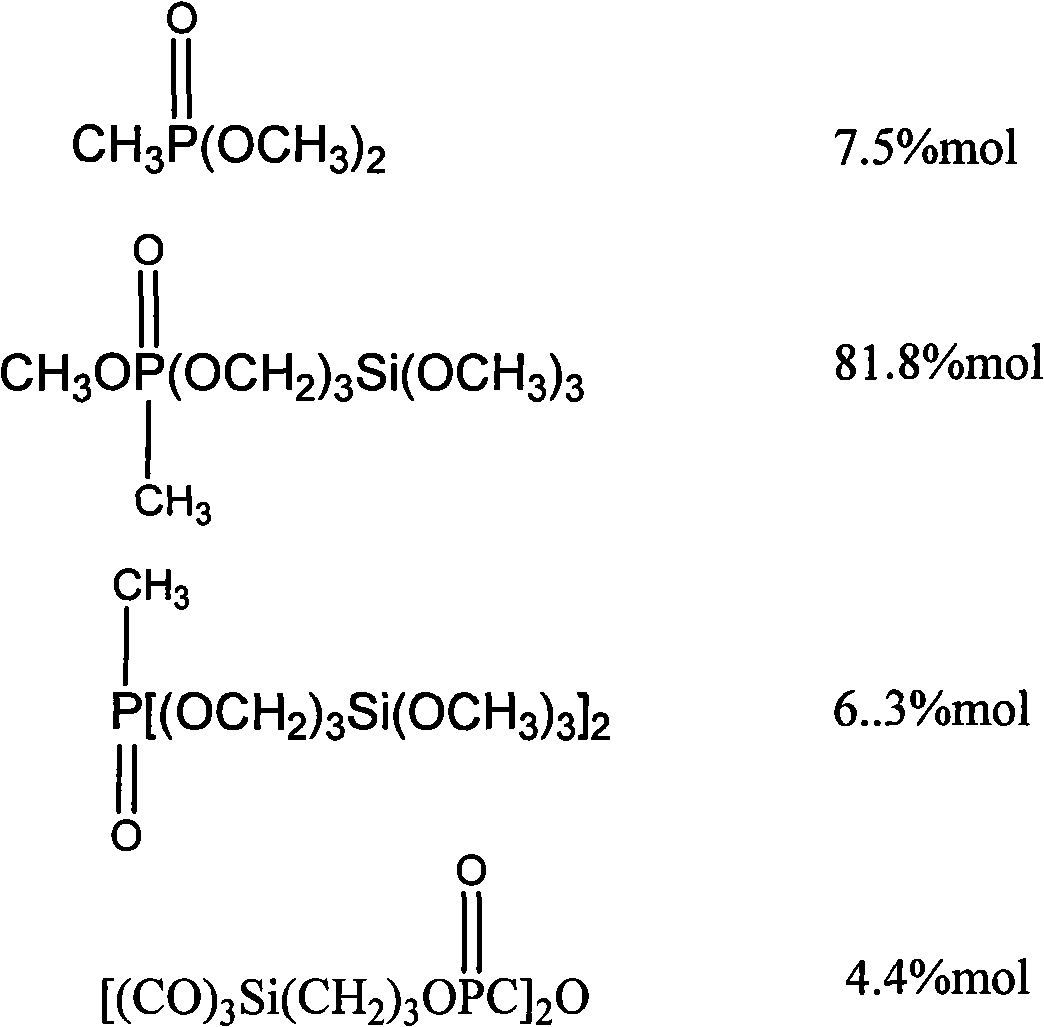Method for preparing stabilizing agent for silicon antifreeze
A technology of antifreeze and stabilizer, which is applied in the preparation of silicon-type antifreeze stabilizer and antifreeze stabilizer. Unable to obtain silicon-type antifreeze, phosphorus-silicon type stabilizer, etc., to achieve the effect of improving selectivity, increasing yield, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In the present embodiment, the preparation method of silicon type antifreeze stabilizer comprises the following steps:
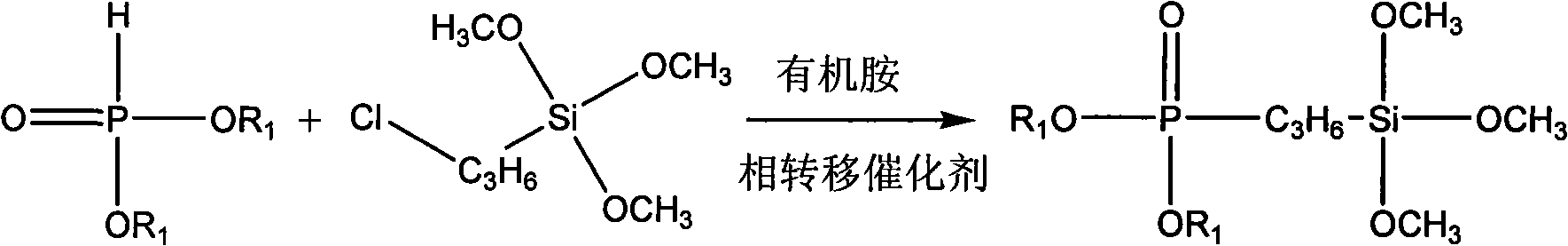
[0026] a. Add 800g of chloropropyltrimethylsilane, 500g of dimethyl phosphate, 6g of n-butylamine and 20g of benzyltriethylammonium chloride into a 2L, 312L stainless steel reaction kettle, press nitrogen to 0.15mpa, Check the airtightness of the equipment, heat and stir, raise the temperature to 120°C, and stir for 4 hours.
[0027] b. Sampling and analysis of the synthesized products. At this time, turn on the nitrogen switch and control the nitrogen flow rate at 0.6L / min. At this time, the temperature of the reactor is controlled at 150°C, and the time for nitrogen flow is 1 hour to remove some low-boiling chlorides and untreated raw materials for the reaction.
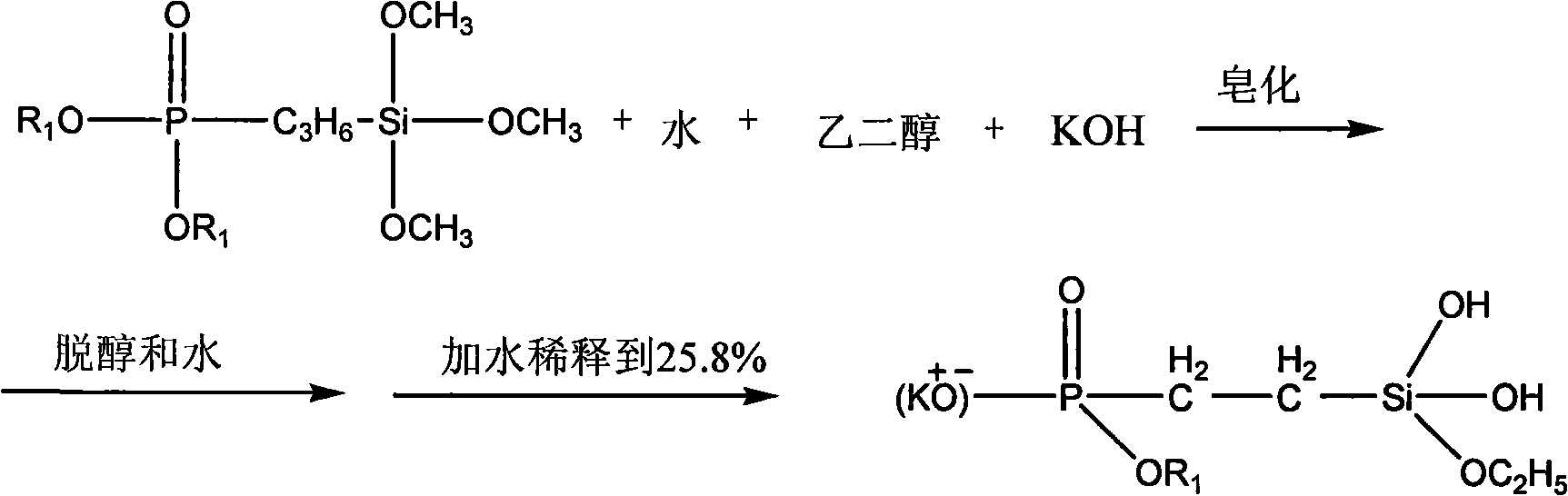
[0028] c. After completion of the chloride removal reaction, use a vacuum oil pump to flash-evaporate the synthesized product, and cut a fraction at 120±20° C. to obtain 800 g of the phos...
Embodiment 2
[0034] In the present embodiment, the preparation method of silicon type antifreeze stabilizer comprises the following steps:
[0035] a. Add 800g of chloropropyltrimethylsilane, 700g of diethyl phosphate, 6g of n-butylamine and 20g of benzyltriethylammonium chloride into a 2L, 312L stainless steel reaction kettle, press nitrogen to 0.15mpa, Check the airtightness of the equipment, heat and stir, raise the temperature to 180°C, and stir for 7 hours.
[0036] b. Sampling and analysis of the synthesized products. At this time, turn on the nitrogen switch and control the nitrogen flow rate at 1.0L / min. At this time, the temperature of the reactor is controlled at 170°C, and the time for nitrogen flow is 2 hours to remove some low-boiling chlorides and untreated raw materials for the reaction.
[0037] c. After completion of the chloride removal reaction, use a vacuum oil pump to flash-evaporate the synthesized product, and cut a fraction at 120±20° C. to obtain 920 g of phosphos...
Embodiment 3
[0043] In the present embodiment, the preparation method of silicon type antifreeze stabilizer comprises the following steps: a. Add 800g chloropropyltrimethylsilane, 1002g dimethyl phosphate, 6g n-butyl in 2L, 312L stainless steel reactor Amine and 20g of benzyltriethylammonium chloride, press nitrogen to 0.15mpa, check the airtightness of the equipment, heat and stir, raise the temperature to 150°C, stir for 6 hours,
[0044] b. Sampling and analysis of the synthesized products. At this time, turn on the nitrogen switch and control the nitrogen flow rate at 0.8L / min. At this time, the temperature of the reactor is controlled at 160°C, and the time for nitrogen flow is 1.5 hours to remove some low-boiling chlorides and untreated raw materials for the reaction.
[0045] c. After completion of the chloride removal reaction, use a vacuum oil pump to flash-evaporate the synthesized product, and cut a fraction at 120±20° C. to obtain 1020 g of the phosphosilane mixture. Yield is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com