Preparation method and application of bencycloquidium bromide optical isomer and composition of bencycloquidium bromide optical isomer
A technology for optical isomers and benzoquinium bromide, which is applied in the field of preparation and application of a group of benzoquine bromide optical isomers and compositions thereof, and can solve the problems of short half-life and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The preparation of embodiment 1 (+)-3-quinoline alcohol and (-)-3-quinoline alcohol
[0021] Dissolve 60 g of 3-quinoline alcohol in 80% ethanol, add 58 g of L(+) tartaric acid, heat and reflux in a water bath for 30 minutes, cool to 45°C, precipitate crystals, filter and recrystallize to obtain (+)-3-quinoline alcohol salt , and the filtrate is set aside. Take the above filtrate, add K 2 CO 3 Adjust the pH to 8, add D(-) tartaric acid, heat to reflux, cool to 45°C, crystallize, filter and recrystallize to obtain (-)-3-quinoline alkoxide. Dissolve (+)-3-quinoline salt and (-)-3-quinoline salt in water, add K 2 CO 3 Adjust the pH to 8, extract with chloroform, combine the extracts, and evaporate the chloroform to dryness to obtain (+)-3-quinoline and (-)-3-quinoline respectively.
Embodiment 2
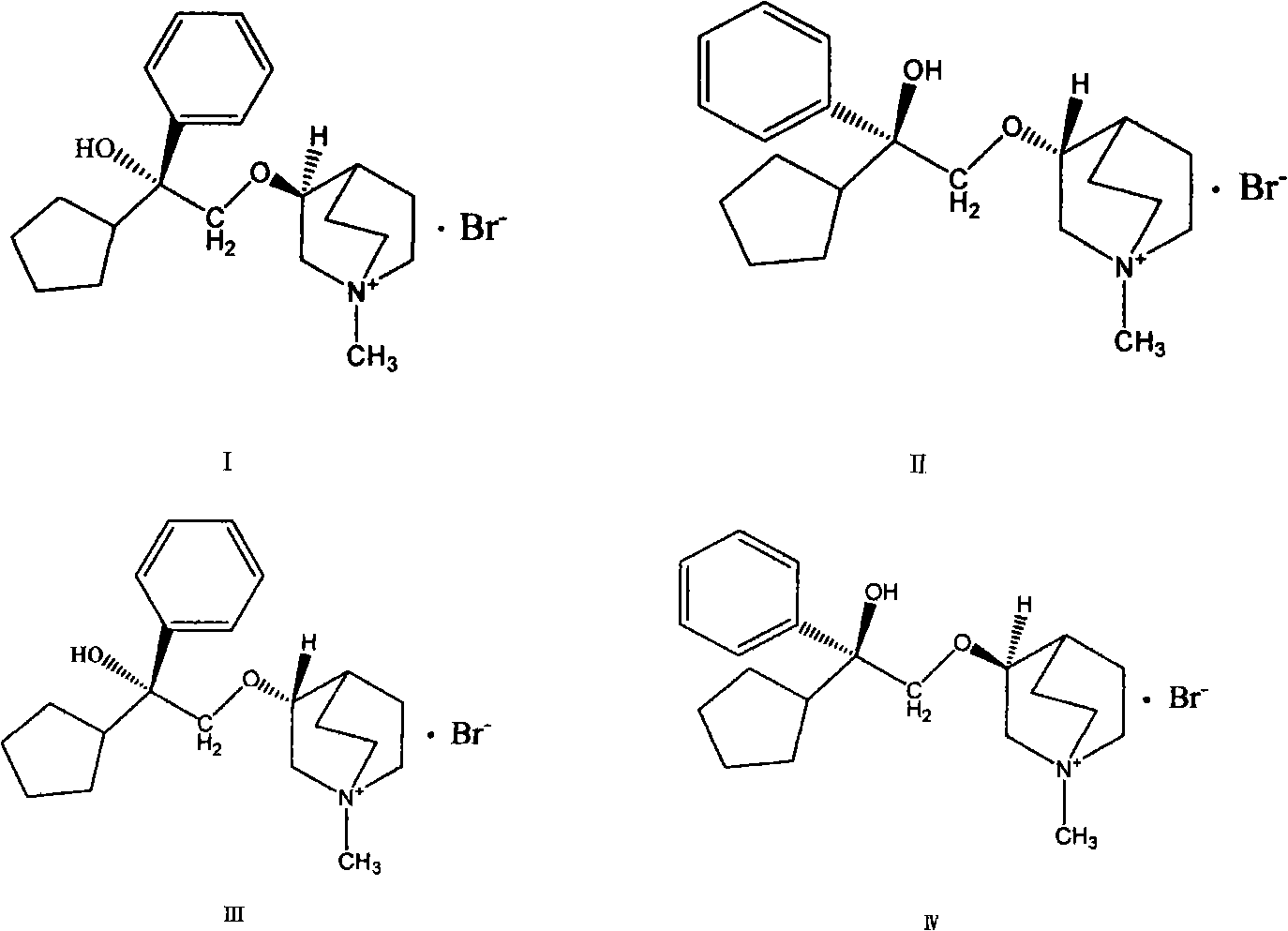
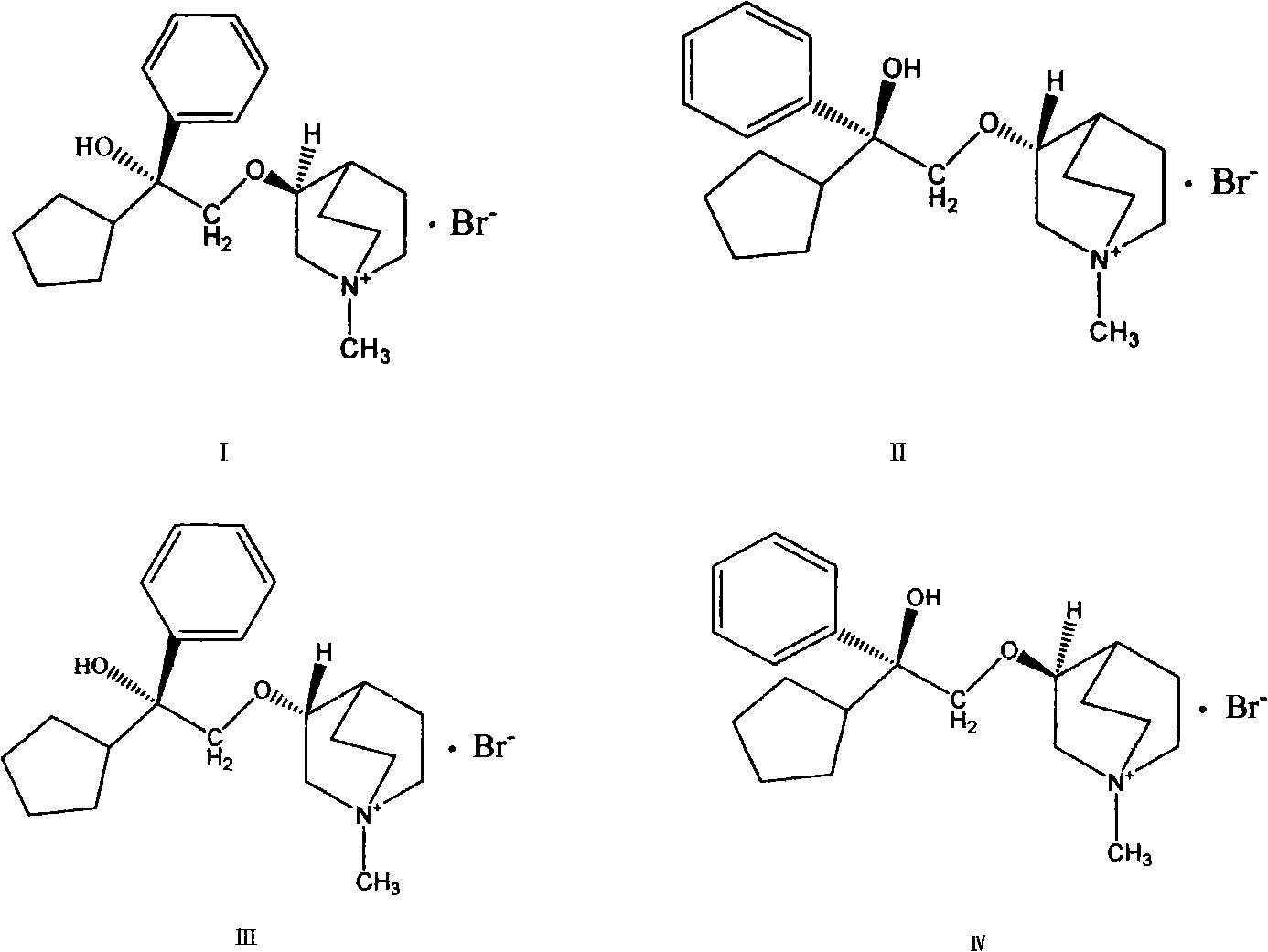
[0022] The preparation of four kinds of pure optical isomers of embodiment 2 phencycloquinium bromide
[0023] Respectively get (+)-3-quinoline alcohol and (-)-3-quinoline alcohol prepared in Example 1 to react with 1-phenyl-1-cyclopentyl oxirane to obtain (+)-pentethyclone Ether mixtures and (-)-penthyclidine mixtures. The (+)-pentehyclidine mixture was subjected to silica gel (100-200 mesh) column chromatography (the eluent was an appropriate proportion of chloroform, methanol, and ammonia water). Take an appropriate amount of (+)-pentehyclidine eluent at different time points, use chloroform / methanol / ammonia water=90 / 10 / 0.02 as the developing solvent, perform TLC analysis, develop color with bismuth potassium iodide reagent, combine the above point and Next point the eluate and evaporate the chloroform to dryness, and react with methyl bromide respectively. Reaction of the upper point with methyl bromide gives the IV isomer, and reaction of the lower point with methyl bro...
Embodiment 3
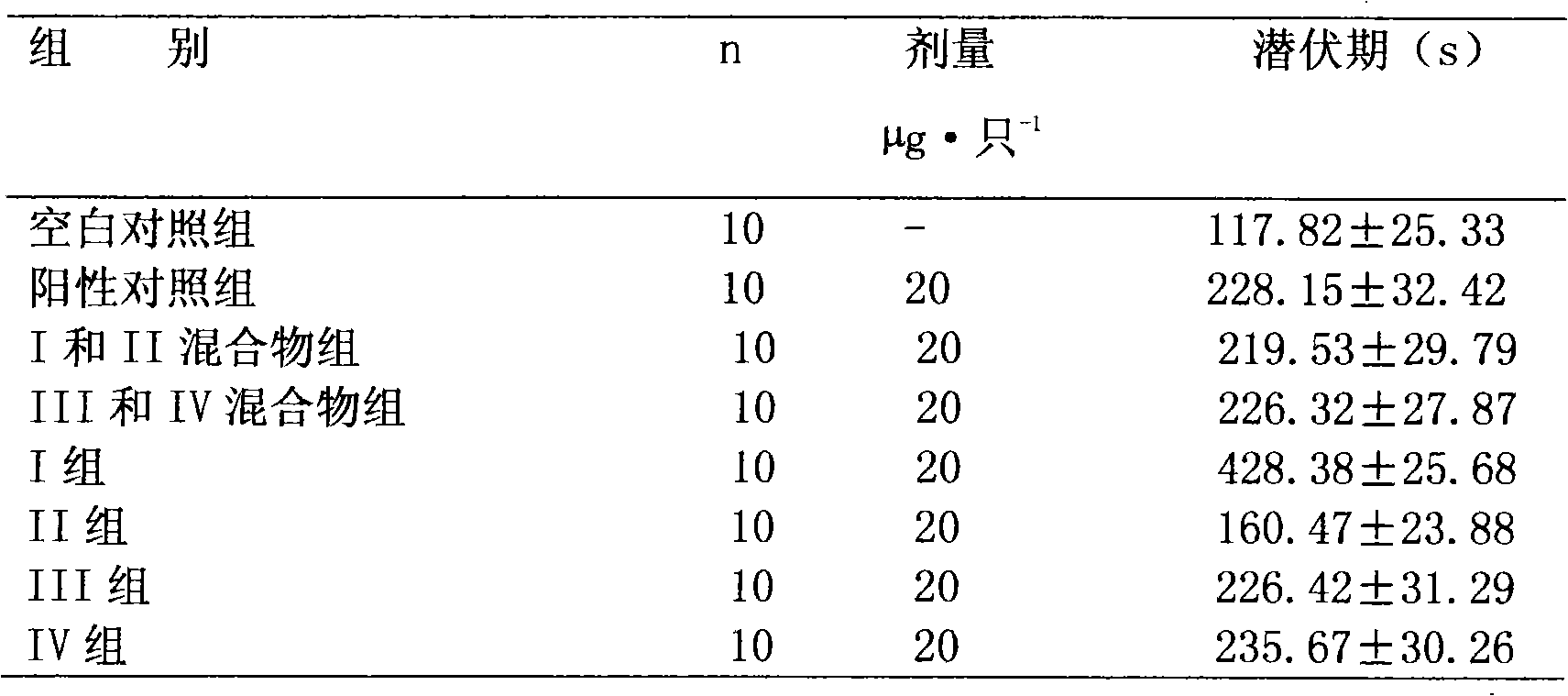
[0038] The preparation of embodiment 3 phencycloquinium bromide isomer I, II mixture and isomer III, V mixture
[0039]Get the (-)-penthyclidine mixture and the (+)-penthyclidine mixture prepared in Example 2 to react with methyl bromide respectively, and the resulting products are respectively isomer I, II mixture and isomer III, V mixture. The two isomer mixtures were quantitatively analyzed by HPLC, and the chromatographic conditions were as follows:
[0040] Chromatographic column: Dikma company C 18 Chromatographic column
[0041] Mobile phase: Phosphate buffer at pH=5.0: Methanol=4:6
[0042] Detection wavelength: 210nm
[0043] Detection results; the purity of the mixture of isomers I and II is 99.5%, and the purity of the mixture of isomers III and V is 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com