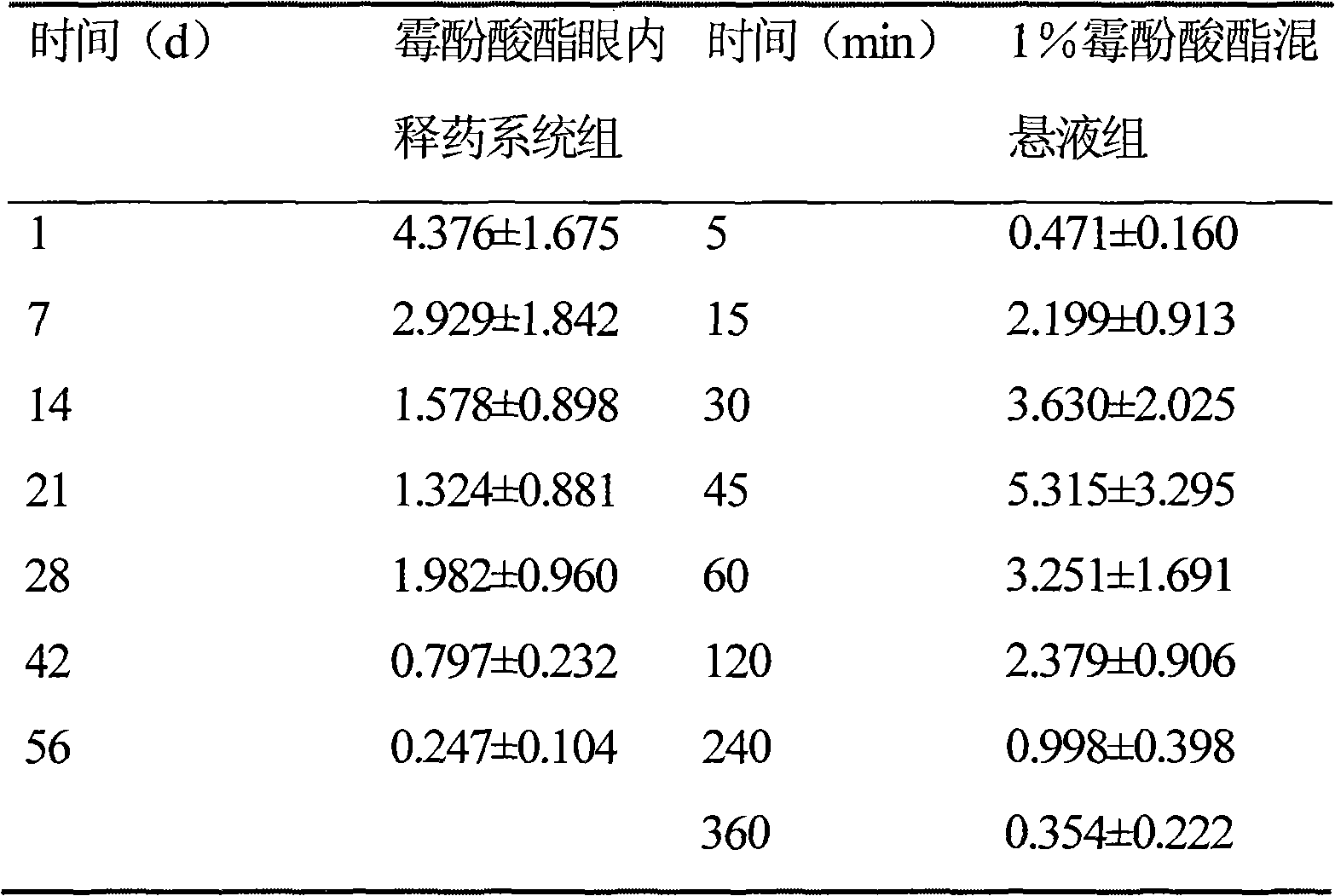
Mycophenolate mofetil intraocular medicament releasing system
A mycophenolate mofetil, drug release technology, applied in the field of long-acting mycophenolate mofetil intraocular sustained release system, can solve the difficulty of penetrating the intraocular drug concentration of mycophenolate mofetil, limit the application of mycophenolate mofetil, and cause serious adverse reactions and other problems, to achieve the effect of reducing the dosage, high drug utilization and long-lasting drug effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Get polylactide-glycolide 250mg of molecular weight 10000 Daltons, add mycophenolate mofetil powder 250mg after dissolving with dichloromethane 5ml (the weight ratio of biodegradable pharmaceutical macromolecule auxiliary material and mycophenolate mofetil 0.5:0.5), stirred and dissolved evenly, poured into a polytetrafluoroethylene mold, placed in a vacuum oven to volatilize dichloromethane. After being completely dried, the polylactide-glycolide drug delivery system containing mycophenolate mofetil was taken off from the polytetrafluoroethylene mold, kept in a vacuum oven at room temperature for 48 hours to completely remove the solvent, and obtained a thickness of 1.5-2 mm sheet-like drug with a pore structure, and then use a die with a 1.5-2 mm aperture to form a mycophenolate mofetil preparation with a thickness of 1.5-2 mm and a diameter of 1.5-2 mm.
Embodiment 2
[0013] According to the method and steps of Example 1, but adopting 400 mg of polylactide-glycolide with a molecular weight of 100,000 Daltons and 10 ml of dichloromethane to dissolve, add 600 mg of mycophenolate mofetil (biodegradable pharmaceutical polymer adjuvant and The weight ratio of mycophenolate mofetil is 0.4:0.6), and a medicine stick with a diameter of 2 mm is prepared, kept in a vacuum oven at room temperature for 48 hours to completely remove the solvent and then cut into 2 mm long mycophenolate mofetil release formulation. The mycophenolate mofetil preparation was sterilized by fumigation with ethylene oxide for 24 hours, and then placed for another week before use.
Embodiment 3
[0015] Get polylactide-glycolide 0.5g of molecular weight 35000 Daltons, add mycophenolate mofetil powder 0.5g after dissolving with dichloromethane 5ml The weight ratio is 0.5:0.5), stirred and dissolved evenly, poured into a polytetrafluoroethylene mold, and placed in a vacuum drying oven to volatilize dichloromethane. After being completely dried, the polylactide-glycolide drug delivery system containing mycophenolate mofetil was taken off from the polytetrafluoroethylene mold, kept in a vacuum oven at room temperature for 48 hours to completely remove the solvent, and obtained a thickness of 1.5-2 mm sheet-like drug with a pore structure, and then use a die with a 1.5-2 mm aperture to form a mycophenolate mofetil preparation with a thickness of 1.5-2 mm and a diameter of 1.5-2 mm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com