Cyclohexane ionic liquid catalyst prepared from benzene and hydrogen and preparation method thereof
A technology of ionic liquid and catalyst, which is applied in the field of benzene hydrogenation to cyclohexane ionic liquid catalyst and its preparation, can solve the problems of hidden safety hazards in production and storage, increased operation difficulty, nitrogen oxide pollution, etc., to reduce temperature, Effects of reduced energy consumption and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
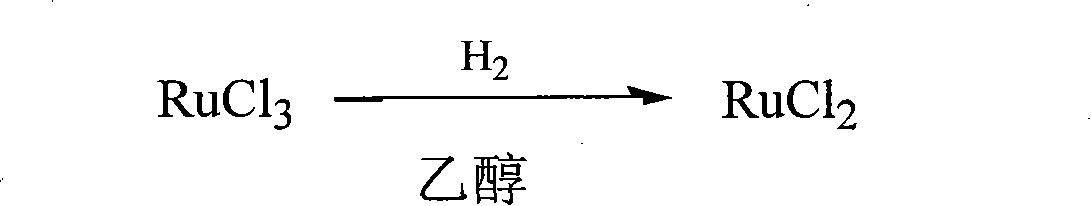
[0020] 1) Take 655mgRuCl 3 ·3H 2 O was added to 50 mL of absolute ethanol, and hydrogen reduction was carried out at 30°C under normal pressure for 46 hours to obtain RuCl 2 ethanol solution, sealed for future use;
[0021] 2) Take 8.210g of N-methylimidazole and 10.183g of n-chlorobutane, add them into a three-necked flask filled with 10mL of ethylbenzene, put them in a constant temperature oil bath, and react for 24h at 106°C and normal pressure. The product was washed with toluene until there was no N-methylimidazole, the detergent was distilled off under reduced pressure, and dried in vacuum at 60°C for 10 h to obtain [BMIM]Cl (16.931 g, yield 96.93%);
[0022] 3) Take the 5mL RuCl prepared in step 1) 2 Ethanol solution and 578mg[BMIM]Cl were placed in a three-necked flask, under normal pressure, N 2 Protected, reacted at 70°C for 6h, distilled under reduced pressure, and dried in vacuo to remove absolute ethanol to obtain the ionic liquid catalyst [BMIM]Cl / [RuCl 2 ],...
Embodiment 2
[0025] 1) Take 65.5mgRuCl 3 ·3H 2 O and 10 mL of absolute ethanol were placed in a high-pressure reactor, and reduced for 30 min at 30 ° C and a hydrogen pressure of 1 Mpa to prepare RuCl 2 ethanol solution, sealed for future use;
[0026] 2) Take 8.210g of N-methylimidazole and 10.183g of n-chlorobutane, add them into a three-necked flask filled with 10mL of ethylbenzene, put them in a constant temperature oil bath, and react for 6h at 106°C and normal pressure. The product was washed with toluene until there was no N-methylimidazole, the detergent was distilled off under reduced pressure, and dried in vacuum at 40°C for 30 hours to obtain [BMIM]Cl (13.427g, yield 76.87%);
[0027] 3) Take the RuCl prepared in step 1) 2 Ethanol solution with 428mg [BMIM]Cl, 300mg ZnCl 2 and 10mL of absolute ethanol, placed in a three-necked flask, under normal pressure, N 2 After protecting and reacting at 78°C for 4 hours, distilling under reduced pressure, and drying in vacuo to remove...
Embodiment 3
[0030] 1) Take 52mgRuCl 3 and 10 mL of absolute ethanol were placed in a high-pressure reactor, and reduced for 20 min at 30 ° C and a hydrogen pressure of 1 Mpa to prepare RuCl 2 ethanol solution, sealed for future use;
[0031] 2) Take 8.210g of N-methylimidazole and 11.11g of n-chlorobutane, add them into a three-necked flask filled with 10mL of ethylbenzene, put them in a constant temperature oil bath, and react for 48h at 88°C and normal pressure. The product was washed with toluene until there was no N-methylimidazole, the detergent was distilled off under reduced pressure, and vacuum-dried at 50°C for 40 h to obtain [BMIM]Cl (13.539 g, yield 77.51%);
[0032] 3) Take the RuCl prepared in step 1) 2 Ethanol solution with 428mg [BMIM]Cl, 300mg ZnCl 2 and 10mL of absolute ethanol, placed in a three-necked flask, under normal pressure, N 2 After protecting and reacting at 78°C for 5.5h, the absolute ethanol was distilled off under reduced pressure to obtain the ionic liq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com