Derivative of acyl thiourea pyridine, method for preparing same and application thereof
A technology of acyl thiouracil and derivatives, which is applied in the field of acyl thiouracil derivatives and their preparation, and can solve the problems of affecting physiological activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
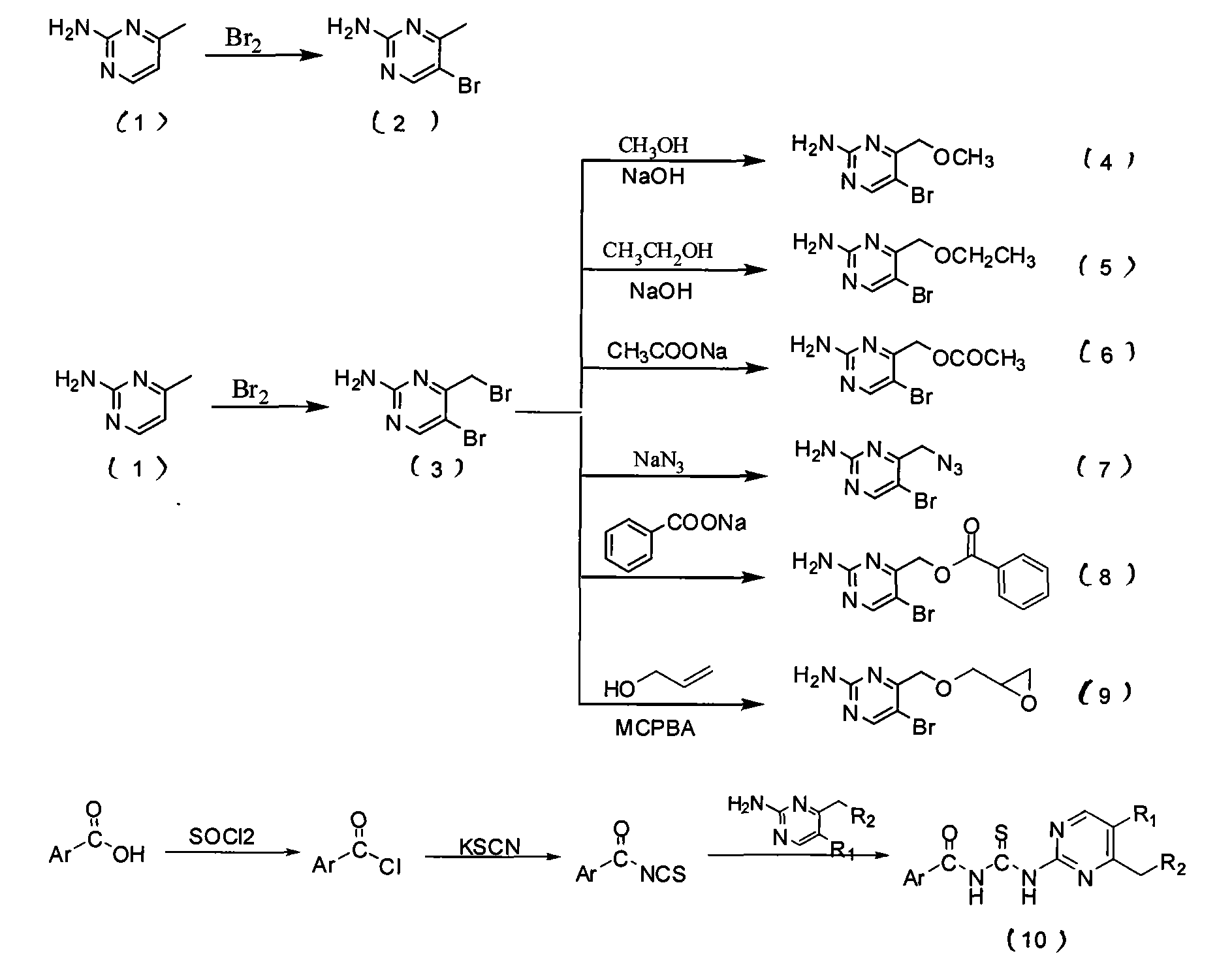
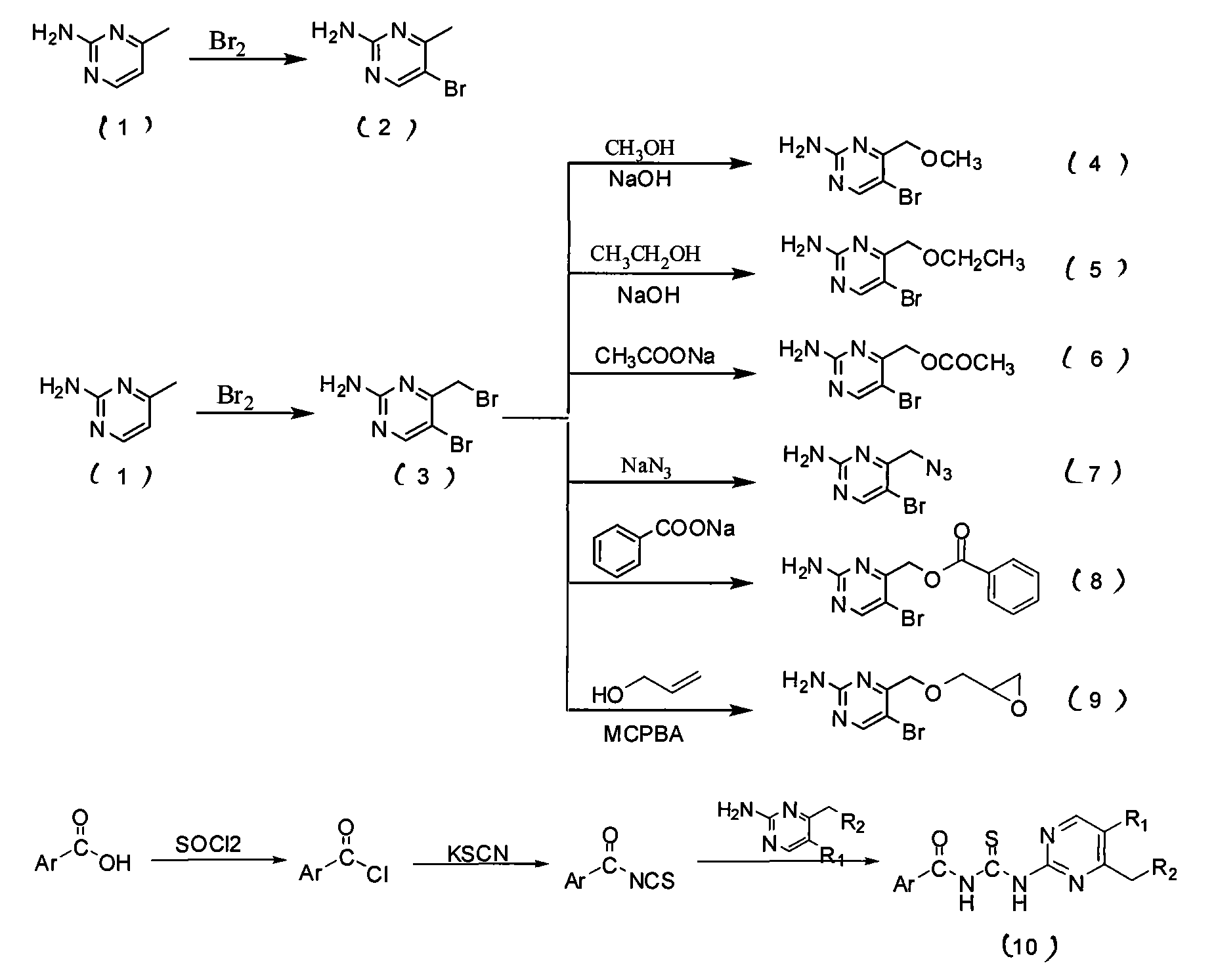
[0042] The preparation of embodiment 1,2-amino-5-bromo-4-bromomethylpyrimidine (compound 2)
[0043] Put 1.09g (10mmol) of compound (1) in a 250mL round bottom flask, add 40mL glacial acetic acid to dissolve, add 0.256mL liquid bromine, and the solution is transparent yellow. Stir at room temperature and monitor by TLC. After the reaction, NaOH and Na 2 CO 3 The pH was adjusted to 7, and a large amount of solid precipitated out. Suction filtration, wash with a large amount of water, transfer the filter cake, and dry to obtain a white solid.
Embodiment 2
[0044] The preparation of embodiment 2,2-amino-5-bromo-4-bromomethylpyrimidine (compound 3)
[0045] Put 1.09g (10mmol) of compound (1) in a 250mL round-bottomed flask, add 40mL of glacial acetic acid to dissolve, add dropwise 0.76mL of liquid bromine, and the solution turns orange-red. After stirring at room temperature, the solution became cloudy after 15 minutes. Continue to stir, and when the solution turns from turbid to clear (about 1 h), quickly add 160 mL of distilled water to obtain a white powdery precipitate and a light yellow supernatant. Filter with suction, wash with a small amount of water, and transfer the filter cake. Methanol-dioxane was recrystallized and dried in vacuo to obtain a white solid.
Embodiment 3
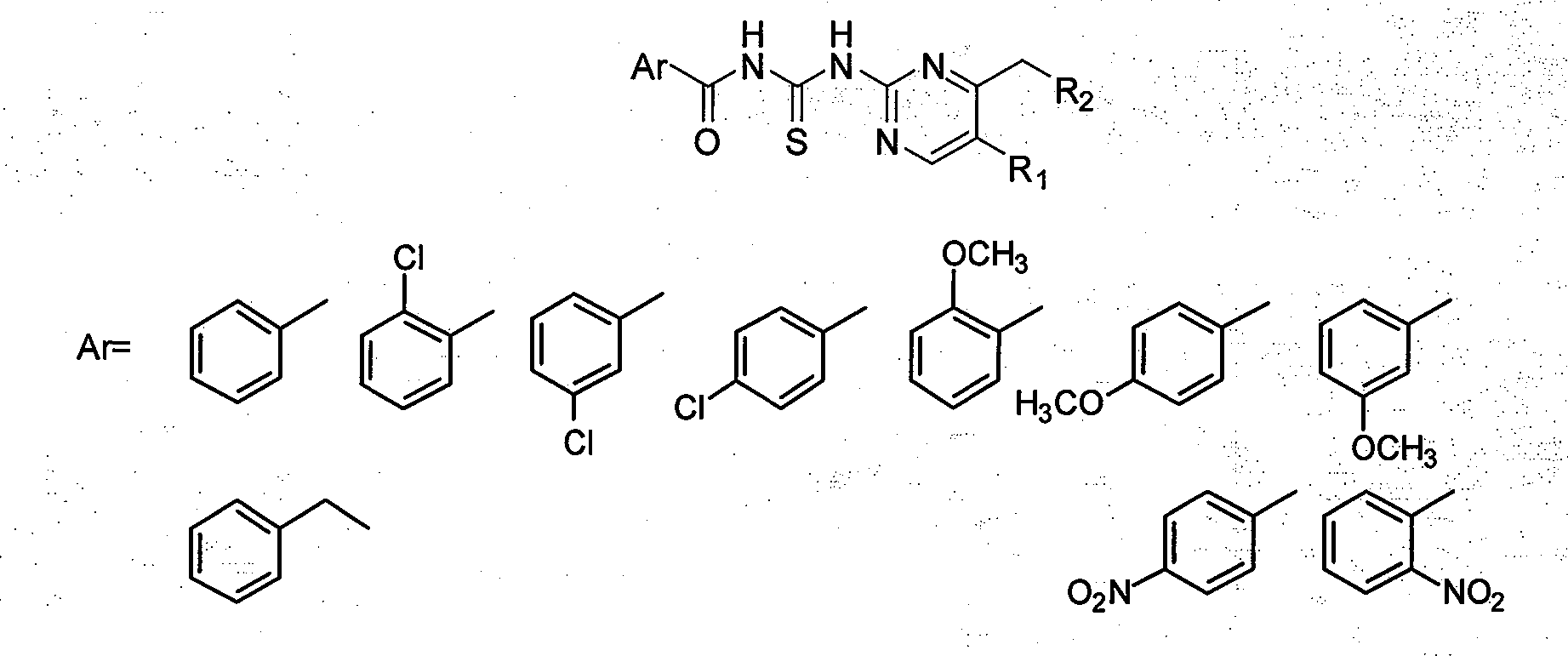
[0046] Embodiment 3, the synthesis of N-[5-bromo-4-phenacylmethylpyrimidin-2-yl]benzoylthiourea
[0047] R 1 =Br
[0048] (1) Preparation of (2-amino-5-bromo-pyrimidin-4-ylmethyl) benzoate (8)
[0049]Add 31.00 g (3.7 mmol) of compound (3) and about 10 mL of DMF to a 100 mL Erlenmeyer flask, and then add 5 mL of an aqueous solution containing 1.60 g (11.1 mmol) of sodium benzoate. Ultrasonic reaction 4h. Add about 50 mL of distilled water, refrigerate and crystallize, filter with suction, wash, and dry in vacuo to obtain a milky white solid. Yield: 75.8%. m.p.130℃~135℃.
[0050] (2) Preparation of benzoyl isothiocyanate
[0051] Add 880mg (9mmol) of KSCN and about 15ml of freshly distilled anhydrous CH to a 100ml eggplant-shaped bottle 3 CN, slowly dropwise into anhydrous CH containing 0.84 mL of benzoyl chloride 3 CN about 10mL, reflux for 2h, and filter with suction. The filtrate was directly carried out to the next reaction without further processing.
[0052]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com