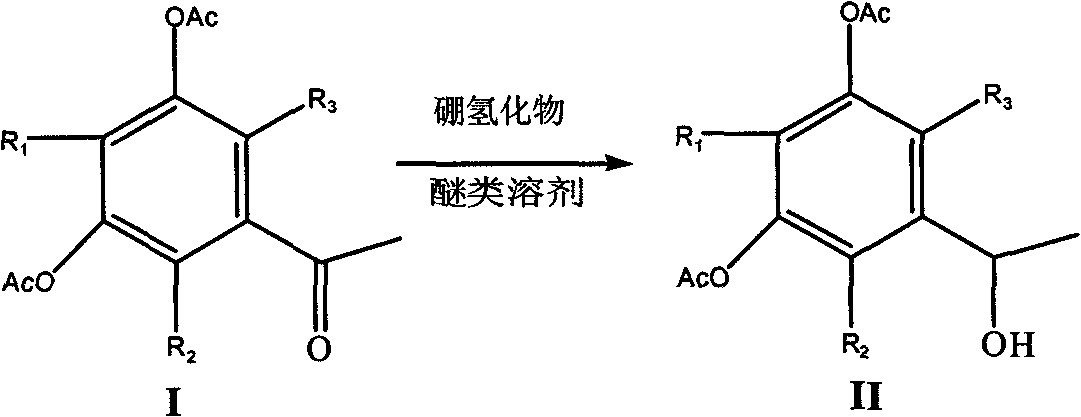
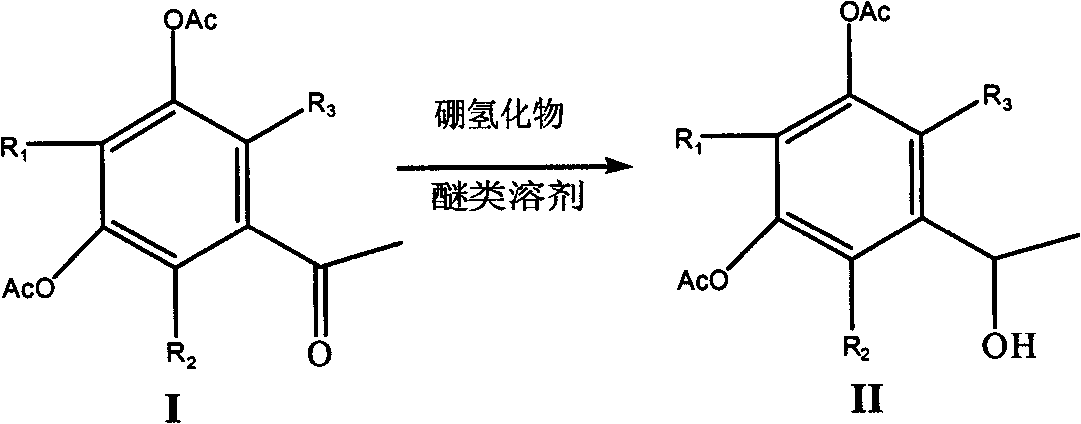
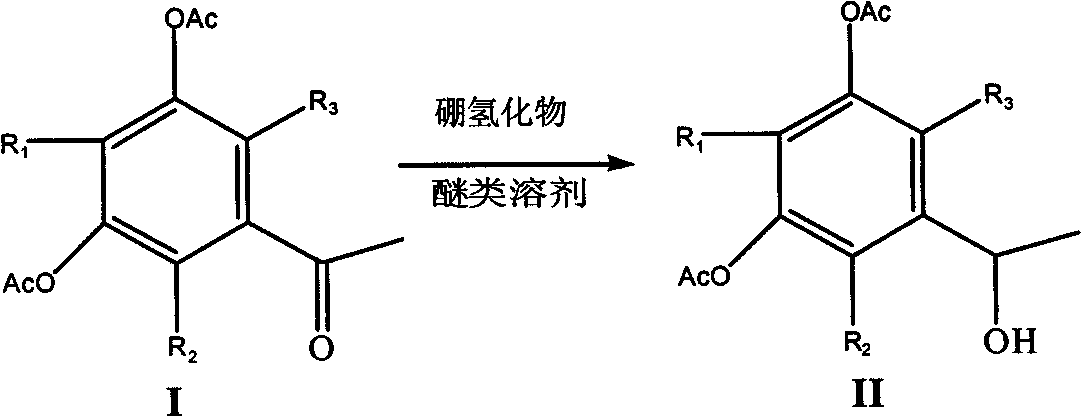
Selective reduction method of ketone group in phenolcarboxylic acid ester
A carboxylic acid phenol ester and selective technology, which is applied in the field of selective reduction, can solve the problems of hidden safety hazards in the use of hydrogen, expensive palladium carbon, etc., and achieve the effects of mild reaction conditions, simple operation, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Dissolve 30g of 3,5-diacetoxyacetophenone in 80ml of tetrahydrofuran, cool to -15°C, slowly add a tetrahydrofuran solution of zinc borohydride (prepared from sodium borohydride and anhydrous zinc chloride), and complete the addition , stirred at -15°C for 24 hours, evaporated the reaction solution to dryness, added 5% sodium hydroxide solution until the pH value was strongly alkaline, extracted twice with 100ml of dichloromethane, washed the combined extracts once with water, dried over magnesium sulfate, and filtered , and the mother liquor was evaporated to dryness to obtain 1-(1-hydroxyethyl)-3,5-diacetoxybenzene as a brownish-yellow liquid with a yield of 70%.
Embodiment 2
[0022] 20g of 2-methyl-3,5-diacetoxyacetophenone in 80ml of tetrahydrofuran, cooled to -15°C, slowly added the tetrahydrofuran solution of zinc borohydride, after the addition was completed, stirred at -15°C for 28 hours, and the reaction The liquid was evaporated to dryness, 5% sodium hydroxide solution was added until the pH value was strongly alkaline, extracted twice with 100 ml of dichloromethane, the combined extracts were washed once with water, dried over magnesium sulfate, filtered, and the mother liquor was evaporated to dryness to obtain the corresponding alcohol, which was collected rate of 65%.
Embodiment 3
[0024] Add 30g of 3,5-diacetoxyacetophenone solvent to 80ml of dioxane, cool to -15°C, slowly add the tetrahydrofuran solution of zinc borohydride, after the addition is complete, stir at -15°C for 24 hours, distill the reaction solution to dry, add 5% sodium hydroxide solution until the pH value is strongly alkaline, extract twice with 100ml of dichloromethane, wash the combined extracts once, dry over magnesium sulfate, filter, and evaporate the mother liquor to dryness to obtain 1-(1-hydroxyethyl Base)-3,5-diacetoxybenzene, brown liquid, yield 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com