Glabrous sarcandra herb extract, quality control method for extract preparation and application of thereof
A quality control method and extract technology, applied in anti-inflammatory agents, pill delivery, suppository delivery, etc., can solve the problems of inability to comprehensively evaluate the quality of the extract of Chinese herbal medicine Scutellaria spondylolis, achieve excellent precision, simple operation method, Highly stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The establishment of the standard fingerprint collection of embodiment 1 Sargassum extract:
[0049] Take 500g of the dried aboveground part of the traditional Chinese medicine Rhizoma sargassum, reflux twice with 10 times the amount of water, two hours each time, combine the extracts, recover the solvent under reduced pressure, freeze-dry after concentration, and obtain 51.2g of Rhizoma sargassum extract.
[0050] Dissolve the above-mentioned standard extract of sargassum with appropriate amount of water to make a solution containing 5.0 mg of sargassum extract per 1 mL of water. After passing through a 0.45 μm filter membrane, the test solution of the sargassum extract is obtained.
[0051] Take the reference substance of rosmarinic acid, dissolve it with an appropriate amount of methanol, and make a solution containing 1 mg of rosmarinic acid per 1 mL of methanol, and obtain the reference substance solution of rosmarinic acid.
[0052] Accurately take 10 μL of the ab...
Embodiment 2
[0053] Embodiment 2 Standard Fingerprint Spectrum Reproducibility Investigation:
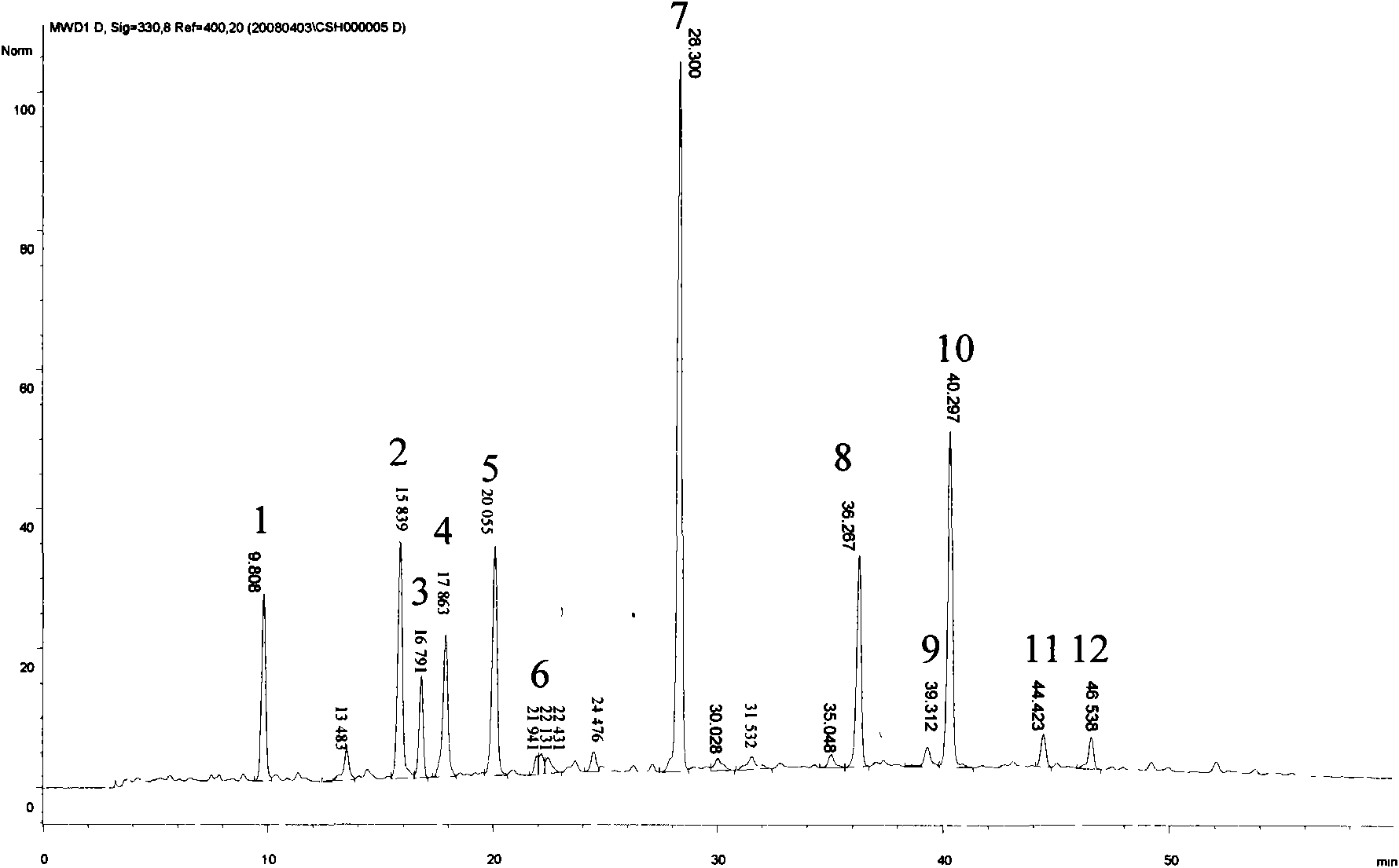
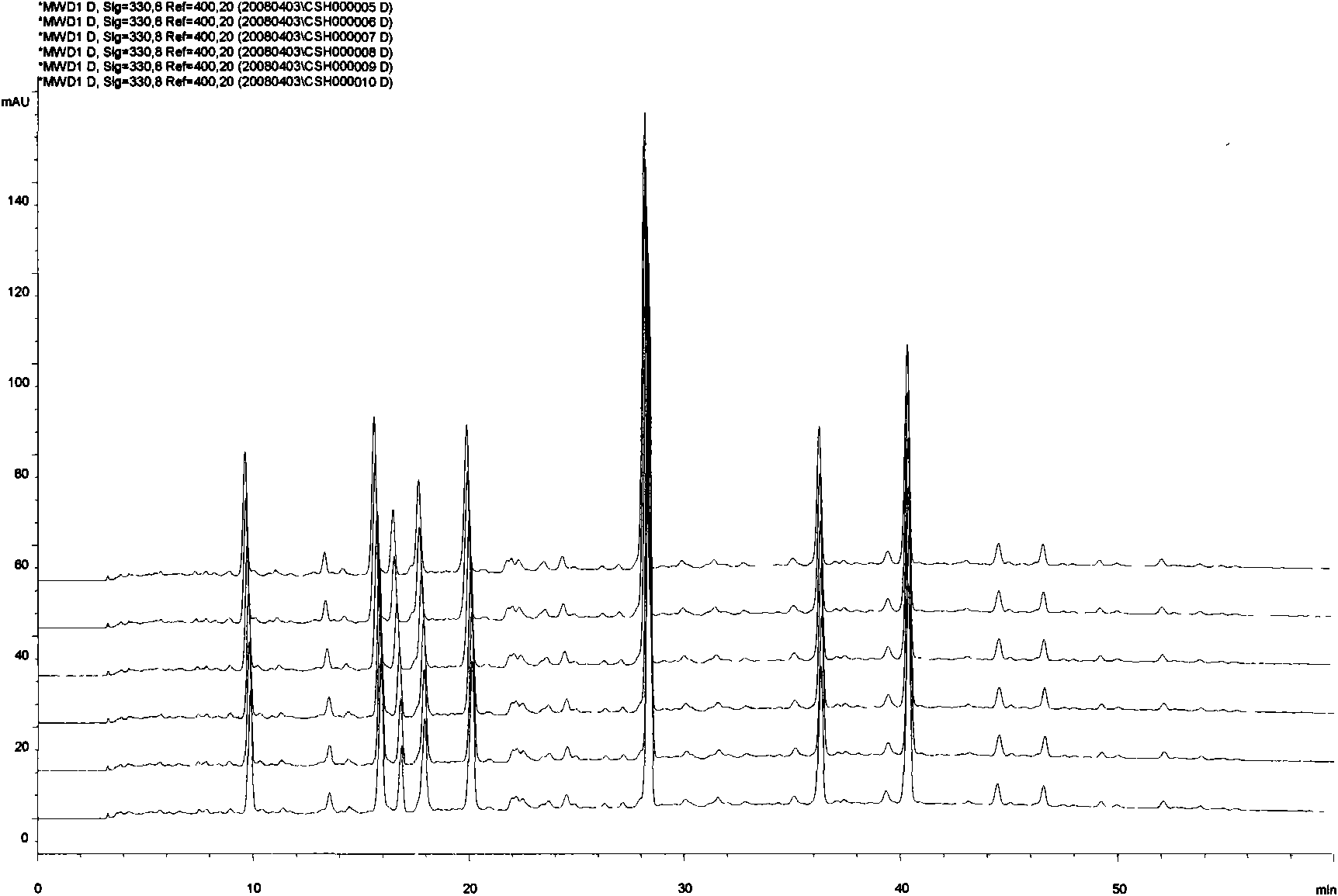
[0054] Accurately weighed 25 mg of Sargassum standard extract in total 6 parts, respectively dissolved in water and fixed volume in a 5mL volumetric flask, after passing through a 0.45 μm filter membrane, took the filtrate as the test solution of Sargassum extract, using Example 1 Carry out chromatographic analysis under the liquid phase condition described in, record chromatogram (see image 3 ). In the HPLC fingerprint spectrum that obtains, the relative retention time of twelve chromatographic peaks all meets the relevant requirement in embodiment 1, and the relative deviation of the relative retention time of each chromatographic peak is all less than 1.0% (seeing table 1), shows that embodiment 1 The obtained HPLC fingerprints have good reproducibility. Utilize the " Chinese medicine chromatographic fingerprint similarity evaluation system 2004A " software calculation provided by Pharmaco...
Embodiment 3
[0057] Enrichment and structural identification of embodiment 3 chromatographic peaks:
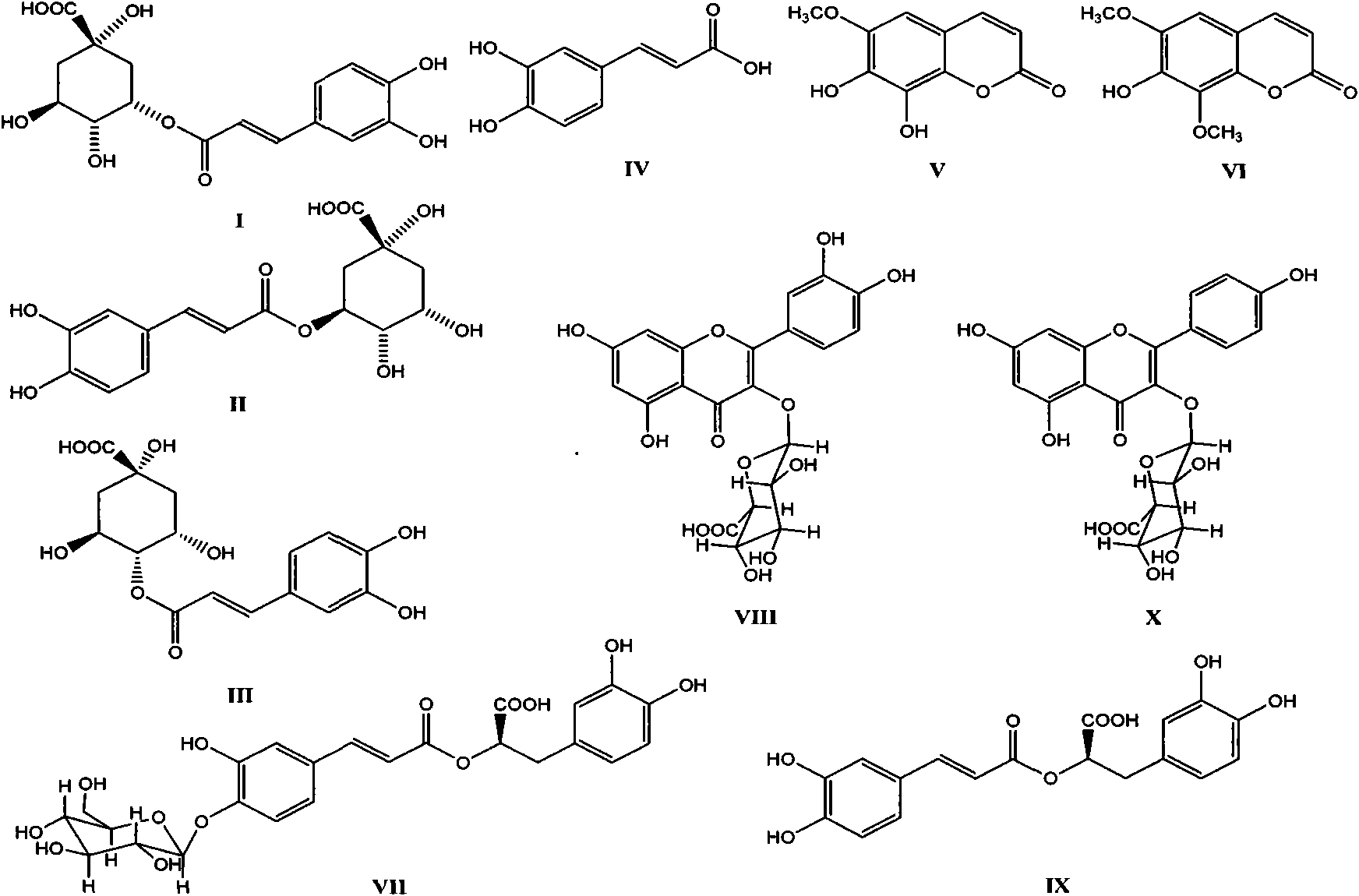
[0058] Take 4kg of the dried aboveground part of the traditional Chinese medicine Zygomyceps sarcophagus, reflux twice with 10 times the amount of water, each time for two hours, combine the extracts, recover the solvent under reduced pressure, freeze-dry after concentration, and obtain 460 g of ZJF extract (ZJF). Suspend it in appropriate amount of water, adopt macroporous resin Diaion Hp-20 open column chromatography, and elute with water, 30% ethanol-water, 50% ethanol-water and 95% ethanol-water respectively. The solvent was recovered under reduced pressure respectively to obtain 340 g of water eluate (ZJF-I), 70 g of 30% ethanol-water eluate (ZJF-II), 50% chromatogram, and methanol-water (10, 30, 50, 100% ) gradient elution. Compounds I, II, and III were obtained from the fraction eluted with 10% methanol under the ODS column (ZJF-IA) by reverse-phase high-efficiency liquid phase sep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com